Cover Crops: Soil Health
Last Updated: 01/22by Clain Jones, Soil Fertility Extension Specialist / Professor; Kathrin Olson-Rutz, Research Associate; Perry Miller, Cropping Systems Professor; Cathy Zabinski, Belowground Ecology Professor; and Susan Tallman, State Agronomist and Certified Crop Adviser

Overview
Cover crops are one tool to improve soil health and long-term agricultural sustainability, especially when grown in place of fallow. Plant residue and living roots are critical for healthy living soils. Microbial activity is largely dependent on the amount of plant residue returned to the soil. In Montana studies, mixed-species cover crops did not create more total biomass (over cover-wheat rotation), thus microbial activity, than single-species pea cover. Cover residue high in N promotes bacterial activity and over time reduces reliance on chemical N fertilizer, thus reducing the risk of soil acidification and nitrate leaching. Specific cover species may provide specific microbial benefits, promote arbuscular mycorrhizal fungi (AM-fungi) or promote disease-suppressing bacteria. However, such specialized benefits are hard to measure.
In semi-arid dryland cropping systems, covers can increase phosphorus (P) and N cycling, improve water infiltration, soil aggregation, and decrease surface soil temperatures. Cover crops tend to reduce subsequent small grain yields in Montana’s semi-arid environment and possibly total organic matter input across rotations. Therefore, covers may not be better than continuous cropping with a diversity of cash crop species to provide plant residue which is a foundation of soil health (1). Terms used in this paper are defined at the end of this document.
Soil Health
A healthy soil is a living system that supports plant growth which in turn supports animals and people. It is teeming with bacteria, fungi and other microbes that provide nutrients and physical support for plant growth and absorbs and holds water. Biologically active soil looks, feels, and smells different from an unhealthy soil (Soil Health Shovel Test), but changes in soil health can be challenging to document.
The abundance of bacteria, fungi, enzymes (a by-product of organic material decomposition by microbial life in the soil), and potentially mineralizable N (PMN; soil organic N that can become plant available by microbial breakdown) are some biological properties used to evaluate soil health. No single property can measure the impact of a change in land management on soil health, but these biological properties may respond before physical and chemical properties (such as available P and pH; 2, 3, both SK).
Some soil properties (e.g., pH, SOM) are routinely provided on soil test results. Biological indicators, such as enzyme activity, are harder to measure, their response may be short-lived (4), and we may not know what a change means to soil health. Increased enzyme activity could mean more microbial activity. It could also mean a certain nutrient is deficient and microbes are excreting more enzyme to extract that nutrient from the SOM.
Individually each soil characteristic may not measurably improve with a change in agronomic practices, yet in combination they indicate improved soil health. Soil health tests, such as Haney and Cornell Comprehensive Assessment of Soil Health are available. Their ‘scores’ relate to potential crop yields when looked at over several years on a given field, but there can be little correlation between scores and yield when looked at in a single year (5, 6).
The shovel test uses subjective rather than measurable indicators of soil health. It is cheap and easy (Soil Health Shovel Test text box, or the USDA NRCS Montana Cropland Soil Health Assessment). Measuring the rate of cotton fabric decomposition is another rapid and easy farm field test of microbial activity (7). See Evaluating Soil Quality and Health and USDA Soil Quality Indicator Sheets for more information on soil health tests.
Soil Health Shovel Test.
| Healthy soil |
|
| Less-healthy soil |
|
Cover crops
Cover crops, also called covers or green manure, are crops grown between cash crops generally for soil conservation purposes. They are usually not intended for harvest, though sometimes annual covers provide feed. Covers are part of a regenerative approach to improve soil health. They increase plant diversity, keep the soil covered, and increase the time that living roots are in the soil. They can provide a variety of benefits to agronomic systems (see Potential Benefits of Cover Crops). Benefits achieved are dependent on many things including soil type, soil moisture, species planted, and when and how the cover is terminated. Whether or not a cover is successful depends on the goal; the goal will determine cover species selection and management. For example, decreasing N needs might result in a different selection than increasing infiltration or soil organic matter. It is unreasonable to expect a cover to provide all the potential benefits.
Potential Benefits of Cover Crops
|
There are several options of when to seed and when and how to terminate covers. This bulletin focuses on covers used as partial- or full-summer fallow replacement in cereal-fallow systems. Some Montana farmers inter- or under-seed covers with the cash crop. Shoulder season covers (after summer harvest to before spring planting) are challenging to establish in much of Montana due to limited soil moisture after cash crop harvest and harsh winter conditions.
Covers for forage (hay or grazed) increase the planting and termination timing options because the forage may offset potential cash crop yield losses. Growing covers full season or seeding them on winter feed grounds once the livestock move off could be feasible. Integrating livestock grazing with covers changes soil water and nutrient dynamics, the goals of covers, and their management and economics. They will be discussed in a future bulletin.
Soil Microbes
An active soil microbial population is the key to healthy soils. It takes plant residue to feed the soil microbes. See Cover Crops: Management for Organic Matter and Nitrogen for residue production. Soils high in soil bacteria have rapid decomposition of plant residue and other organic matter. Soil fungi tend to increase SOM stability and soil aggregation (8, PA). Arbuscular mycorrhizal fungi are a subgroup of fungi that help plants take up water and nutrients through a symbiotic relationship with plant roots. In conjunction with living plant roots, these fungi produce sugars and proteins that coat soil particles to form soil aggregates (9). There are frequent new discoveries on other ways by which the soil microbial community helps soil health and plant production.
Covers often increase both fungi and bacteria abundance. However, based on work with potatoes, certain microbes may only be associated with certain covers (10, CO). Some plants promote AM-fungi (e.g., oat, cereal rye, clover) and others are associated with non AM-fungi (e.g., hairy vetch; 8, PA).
Most of the indices used to measure soil biological activity were significantly improved over fallow after three rotations (six years) of continuous wheat, and even more with legume covers in place of fallow (2, SK). During the wheat phase, the number of bacteria and fungi (Figure 1), microbial biomass C, and microbial biomass N were 1.7 to 3.9 times higher in legume cover crop than fallow-wheat. These microbiological attributes were far more responsive to crop rotation than the rate of N and C mineralization, total soil organic N and C, or soil aggregate stability (11).
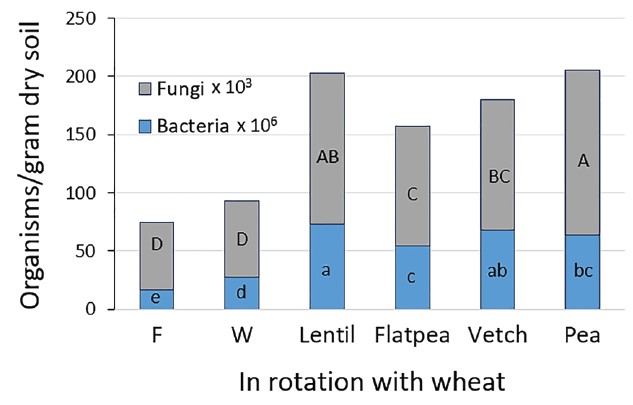
Figure 1. Fungi and bacteria counts in top 4-inch of soil following six years of crop rotations. F – fallow, W – spring wheat. Sampled in October, two months after wheat harvest, 15 months after cover crop termination. Bars with at least one same letter within microbe group are equal with 95% confidence (2, SK).
Microbial activity increases with plant residue input and N concentration of the residue. In an 18-year Montana study, three of the four enzymes used to measure microbial activity were correlated with above ground biomass (12). In an 8-year Montana study of partial fallow covers planted in May and terminated at early bloom, microbial biomass and activity was greater in some years after covers than fallow at spring seeding the following year. Microbial populations did not increase with increasing number of cover rotations (13, MT). Since early terminated covers may not produce as much biomass as cash crop stubble in semi-arid conditions, they are likely not as good as re-crop at feeding microbial populations. Terminating covers before plant maturity (lower C:N, Figure 2), and including legumes in the cover are ways to increase residue N and microbial activity (15, MT).
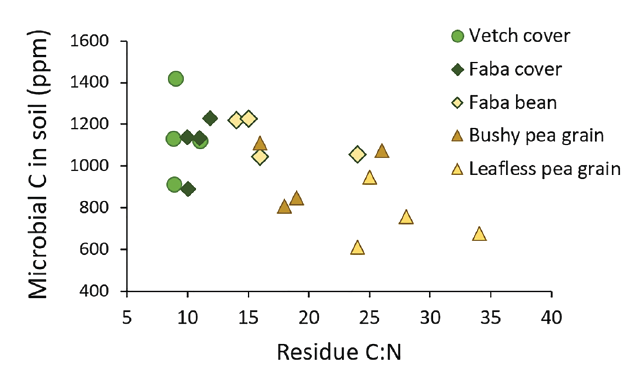
Figure 2. Relationship between the initial C:N of crop residue and soil microbial biomass C in summer of third subsequent crop (14, AB).
Many of the benefits of covers can be attributed to increased plant diversity, which is important to fully functioning ecosystems and soil health. A few studies have been able to document greater bacterial diversity with mixed covers rather than single species covers (16, SD; 17, ID). Microbial activity appears to be more dependent on residue biomass (18, MT; 16, SD) than diversity (17, ID).
With growing interest in regenerative agriculture, there is more focus and question about AM-fungi (19). In a South Dakota study with oat, canola and vetch, only oat cover or mixes containing oat had enough AM-fungi in late fall to increase AM-fungi root colonization and growth of the following crop (20). Brassicas may actually reduce AM-fungi, but promote disease-suppressing bacteria (21). After 8-years (four cover-wheat rotations), wheat following brassica cover had less AM-fungi than following a cover of grasses, but overall fields with covers did not consistently have more AM-fungi than fallow (13, MT).
A more direct way to sustain AM-fungi across rotations is to interplant AM-fungi-supporting covers with the non- supporting cash crop (e.g., canola). For example, when red clover was sown between cabbage rows (non AM-fungi hosting) the following winter wheat yields increased (22, Japan).
Arbuscular mycorrhizal fungi require some soil N. Since 45 lb N/acre added as ammonium nitrate increased AM-fungi, (23), AM-fungi should benefit directly from soil N provided by legumes. The AM-fungi can also benefit indirectly from legumes if less ammonium-based N fertilizer is applied because of legume supplied N. Fungi are sensitive to soil acidity. With more than 45 lb N/acre as ammonium nitrate, AM-fungi decreased due to local soil acidification by the fertilizer.
Arbuscular mycorrhizal fungi biomass decreased by about 40% as pH dropped from 7.3 to 6.1 (23). Such drops in soil pH are being seen in Montana’s traditionally high pH croplands as a result of decades of ammonium-based N fertilization (MSU Extension: Cropland Soil Acidification).
In semi-arid systems with low biomass production, soil biological response from covers is hard to measure with just a ‘few’ rotations, especially in plot studies where field sampling activity can easily carry microbes from one treatment to the next. Soil biology is highly year dependent and it may be cumulative. Also, cover effect may be easily overridden by another factor such as drought, pesticide, fertilizer, or soil pH (24).
Chemical
Soil organic carbon (~60% of SOM), organic N, nitrate-N, P, and pH are some of the chemical indicators of soil’s capacity to store and provide nutrients. Soil organic matter and N are discussed in Cover Crops: Management for Organic Matter and Nitrogen.
Phosphorus acquired by crops is recycled annually through above and below ground plant residue decomposition. An Alberta study compared decomposition of residue from red clover and pea covers, and mature field pea, canola and wheat in fields in their seventh and eighth year of tillage or no till (25). The clover and pea cover residue provided equal or more P and provided it earlier in the next growing season than the mature pea, canola and wheat crop residues. Only the covers released significant amounts of available P for the next crop (10 to 11 lb P2O5/acre). In contrast, mature canola, pea and wheat stubble residue released less than 2 lb P2O5/acre. Most of the P was released within the first 10 weeks after termination or harvest, whether the field was tilled or not.
Cover crop composition (1, 2, 6 and 8-species) did not affect Olsen P after two cycles in Montana (26). However, grasses are well suited to catch P (and N) runoff or deep seepage in acidic or sandy soils, which do not bind P (27, Australia; 28). In contrast, brassicas released more P than other species after freeze-thaw cycles, making the P vulnerable to runoff loss in the spring (29).
Soil acidification in the top few inches is an emerging soil health issue in Montana croplands, leading to reduced yield and crop failure. Without plant cover, the soil is vulnerable to erosion. A major contributing factor is use of ammonia based N fertilizer (MSU Extension: Cropland Soil Acidification). Legume covers should help prevent soil acidification by reducing reliance on commercial N fertilizer and N leaching. In Montana studies with covers, soil pH changed more based on the amount of fertilizer N applied than the type of covers in rotation (30). Pea cover, pea grain and fallow followed by wheat all received less N and had less soil acidification than continuous wheat over nine rotations (18 years, Figure 3).
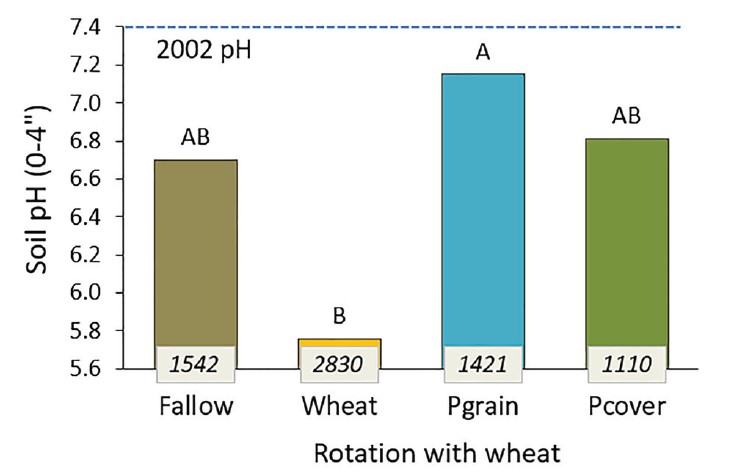
Figure 3. Soil pH after 18 years of fallow, wheat, pea grain, pea cover in no-till rotation with wheat, and total N fertilizer (lb N/ acre) applied to each system. Bars with the same letters are equal with 90% confidence (30).
Physical
Soil aggregation increases the soil’s ability to absorb and hold water and resist wind and water erosion. It is a result of complex interactions between the soil microbial community (e.g., fungi, bacteria), soil micro fauna (e.g., worms, nematodes), plant roots, and cementing/binding agents, all of which benefit from covers (31). Wet aggregate stability (also called water stable aggregates) increased with residue input (15, MT). In a Saskatchewan study, wind erodibility after six years decreased due to larger soil aggregates. Legume cover-wheat had the largest soil aggregates, fallow-wheat the smallest, and continuous wheat was intermediate (11). In contrast, aggregate stability did not increase after two rotations of cover, rather than fallow-wheat, at four Montana sites (32). Bulk density and penetration resistance were not affected by cropping system in either the Saskatchewan or Montana study.
Water infiltration is highly variable across a field; therefore, it is hard to document changes with management at least in shorter-term studies. After seven years there was no difference in infiltration between cover and fallow-wheat (26, MT). In a different study, after 18 years, fallow had the lowest infiltration; the other rotations, including cover and re-crop, had higher infiltration rates with some indication that infiltration increases with SOM (Figure 4).
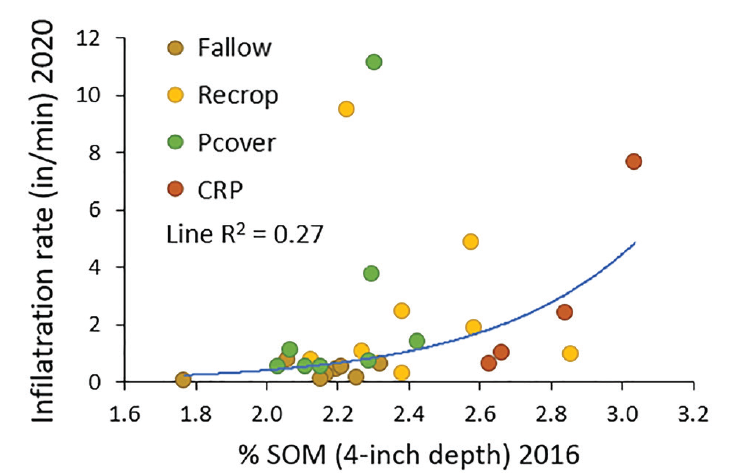
Figure 4. Water infiltration rate by % SOM after 18 years of fallow, wheat or pea grain (re-crop), pea cover (chemical terminated generally at plump pod, sooner if weeds were a problem, or hayed then sprayed) in rotation with wheat, and CRP (10 years Conservation Reserve Program: mostly alfalfa with some grass) followed by pea grain-wheat (eight years; 33, 30, MT).
Soil temperature at a 2-inch depth was lower by 5 to 15°F with covers than fallow from late June through late August (the last temperature measurement) even well after cover termination (Figure 5). Lowered summer afternoon temperatures should decrease evaporation and likely benefit biological activity.
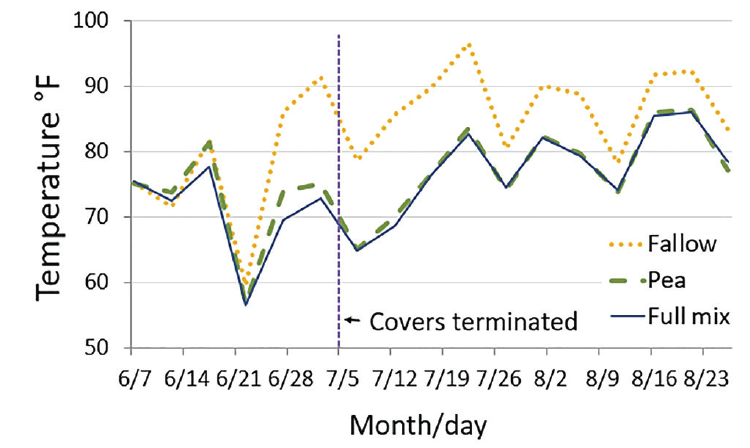
Figure 5. Summer soil temperature at 2-inch depth at 4 p.m. in fallow, pea cover, and 8-species (Full) cover chemically terminated at early bloom (32, MT).
Conclusion
Above and below ground plant residue is critical for healthy living soils, and for that reason fallow is counterproductive to soil health. Plant residue is required to keep the soil microbial community alive, to improve water infiltration and soil aggregation, and to increase N, P, and carbon cycling. Producers need to be clear on the intended goal of planting a cover. In semi-arid dryland systems, a good place to start regenerating soil health is to reduce fallow and increase the frequency of live plant cover and residue on a given field. Including legumes in covers increases plant residue N, which increases microbial activity and the soil’s N supply.
Acknowledgements
We thank the Western Sustainable Agriculture Research and Education (WSARE) program and the Montana Fertilizer Advisory Committee for funding MSU studies. We appreciate the time and expertise of the following for review and production of this bulletin: Kari Lewis (MSU Extension agent), Luke Ahlman (Certified Crop Adviser), Carl Vander Molen (Producer), and MSU Communications.
References
1. Engel, R.E., P.R. Miller, B.C. McConkey, R. Wallander, and J.A. Holmes. 2017. Soil organic C changes to ten years of increasing cropping system intensity and no-till in a semiarid climate.Soil Sci. Soc. Am. J. 81:404-413. https://doi.org/10.2136/sssaj2016.06.0194
2. Biederbeck V.O., R.P. Zentner, and C.A. Campbell. 2005. Soil microbial populations and activities as influenced by legume green fallow in a semiarid climate. Soil Biol. Biochem. 37:1775- 1784. https://doi.org/10.1016/j.soilbio.2005.02.011
3. Liebig, M., L. Carpenter-Boggs, J.M.F. Johnson, S. Wright, andN. Barbour. 2006. Cropping system effects on soil biological characteristics in the Great Plains. Renewable Agric. Food Syst. 21:36-48. https://doi.org/10.1079/RAF2005124
4. Kim, N., M. Zabaloy, K. Guan, and M. Villamil. 2020. Do cover crops benefit soil microbes? A meta-analysis of current research. Soil Biology and Biochemistry. 142. https://doi.org/10.1016/j.soilbio.2019.107701
5. Crookston, B., M. Yost, M. Bowman, K. Veum, and G. Cardon. 2021. How Often do Soil Health Assessment Scores Relate to Crop Yield? Soil Health Innovations Conference. March 2021. NCAT-ATTRA
6. Jones, C., and D. Boss. 2014. Unpublished data
7. Sanyal, D., J. Wolthuizen, and A. Bly. 2020. Cotton Strip Soil Test:Rapid Assessment of Soil Microbial Activity and Diversity in the Field. South Dakota State University Extension https://extension.sdstate.edu/cotton-strip-soil-test-rapid-assessment-soil-microbial-activity-and-diversity-field
8. Finney, D.M., J.S. Buyer, and J.P. Kaye. 2017. Living cover crops have immediate impacts on soil microbial community structure and function. J. Soil Water Conserv. 72:361-373. https://doi.org/10.2489/jswc.72.4.361
9. Hoorman, J., J.C. de Moraes Sá, and R. Reeder. 2009. The Biology of Soil Compaction. Ohio State University Extension SAG-10-09 AEX-543-09. https://ohioline.osu.edu/factsheet/SAG-10
10. Barnett, B.A., J.A. Delgado, and D.K. Manter. 2012. Effects of cover crops on the microbial community and its ability to suppress disease and acquire nutrients. Potato Association of America Proceedings. p. 10.
11. Biederbeck, V.O., C.A. Campbell, V. Rasiah, R.P. Zentner, andG. Wen. 1998. Soil quality attributes as influenced by annual legumes used as green manure. Soil Biol. Biochem. 30:1177- 1185. https://doi.org/10.1016/S0038-0717(97)00150-8
12. Fouts, W. Impacts of crop rotations and nitrogen fertilizer on soil biological factors in semi-arid Montana. MontanaState University Professional Paper. https://scholarworks.montana.edu/xmlui/handle/1/16425
13. Housman, M., S. Tallman, C. Jones, P. Miller, and C. Zabinski. 2021. Soil biological response to multi-species cover crops in the Northern Great Plains. Agric. Ecosyst. Environ. 313:107373. https://doi.org/10.1016/j.agee.2021.107373
14. Lupwayi, N.Z. and Y.K. Soon. 2016. Soil microbial properties during decomposition of pulse crop and legume green manure residues in three consecutive subsequent crops. Can. J. Soil Sci. 96:413-426. https://doi.org/10.1139/cjss-2016-0039
15. O’Dea, J.K., C.A. Jones, C. Zabinski, P.R. Miller, and I.L. Keren. 2015. Legume, cropping intensity, and N-fertilization effectson soil attributes and processes from an eight-year-old semiarid wheat system. Nutr. Cycle. Agroecosys. 102:179-194. https://doi.org/10.1007/s10705-015-9687-4
16. Sanyal, D., J. Wolthuizen, A. Bly, A. Rahhal, and H. Bielenberg. 2021. Cover Crops towards Soil Health Improvements. Soil Health Innovations Conference. March 2021. NCAT-ATTRA
17. Sone, B., J. Lucas, and M. Strickland. 2021. Cover Crop Diversity Affects Microbial Community Diversity and Soil Carbon. Soil Health Innovations Conference. March 2021. NCAT-ATTRA
18. D’Agati, K. 2020. Soil Response to Long Term Multispecies Cover Crop Mixes and Above Ground Biomass in the Semi- arid Northern Great Plains. MS Thesis. Montana State University. 112 p. https://scholarworks.montana.edu/xmlui/handle/1/16036
19. Karas, S. 2020. How the Fungus Might Save Us. California State University, Chico. Center for Regenerative Agriculture and Resilient Systems. https://www.csuchico.edu/regenerativeagriculture/blog/how-fungus-might-save-us.shtml
20. Lehman, R., W.I. Taheri, S.L. Osborne, J.S. Buyer, and D.D. Douds, Jr. 2012. Fall cover cropping can increase arbuscular mycorrhizae in soils supporting intensive agricultural production. Appl. Soil Ecol. 61:300-304. https://doi.org/10.1016/j.apsoil.2011.11.008
21. Vukicevich, E., T. Lowery, P. Bowen, J.R. Úrbez-Torres, and M.Hart. 2016. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review.Agron. Sustain. Dev. 38:48. https://doi.org/10.1007/s13593-016-0385-7
22. Karasawa, T., and M. Takebe. 2012. Temporal or spatial arrangements of cover crops to promote arbuscular mycorrhizal colonization and P uptake of upland crops grown after nonmycorrhizal crops. Plant and Soil. 353:355-366. https://doi.org/10.1007/s11104-011-1036-z
23. Pan, S., Y. Wang, Y. Qiu, D. Chen, L. Zhang, C. Ye, H. Guo,W. Zhu, A. Chen, G. Xu, Y. Zhang, Y. Bai, and S. Hu. 2020. Nitrogen-induced acidification, not N-nutrient, dominates suppressive N effects on arbuscular mycorrhizal fungi. Global Change Biology. https://doi.org/10.1111/gcb.15311
24. Tosi, M., E. Mitter, J. Gaiero, and K. Dunfield. 2020. It takes three to tango: the importance of microbes, hostplant, and soil management to elucidate manipulation strategies for the plant microbiome. Can. J. Microbiol. 66:413-433. http://dx.doi.org/10.1139/cjm-2020-0085
25. Lupwayi, N.Z., G.W. Clayton, J.T. O’Donovan, K.N. Harker,T.K. Turkington, and Y.K. Soon. 2007. Phosphorus release during decomposition of crop residues under conventional and zero tillage. Soil Tillage Res. 95:231–239. https://doi.org/10.1016/j.still.2007.01.007
26. Jones, C. Unpublished data
27. Alamgir, M., and P. Marschner. 2016. Changes in P pools over three months in two soils amended with legume residues. J. Soil Science and Plant Nutrition. 16:76-87. https://doi.org/10.4067/S0718-95162016005000006
28. Hallama, M., C. Pekrun, H. Lambers, and E. Kandeler. 2019. Hidden miners - the roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant and Soil. 434:7-45. https://doi.org/10.1007/s11104-018-3810-7
29. Liu, J., M. Macrae, J. Elliott, H. Baulch, H. Wilson, and P. Kleinman. 2019. Impacts of cover crops and crop residues on phosphorus losses in cold climates: a review. J. Environ. Qual. 48:850-868. https://doi.org/10.2134/jeq2019.03.0119
30. Jones, C., and P. Miller. Unpublished data
31. Lal, R. 2015. Soil carbon sequestration and aggregation by cover cropping. J. Soil Water Conserv. 70:329-339. https://doi.org/10.2489/jswc.70.6.329
32. Miller, P., C. Jones, C. Zabinski, J. Norton, S. Tallman, andM. Housman. 2016. Using cover crop mixtures to improve soil health in low rainfall areas of the northern plains. USDA WSARE Final Report. 40 pp. https://projects.sare.org/sare_project/sw11-099/
33. Ewing, S., P. Miller, C. Jones. Unpublished data.
Resources
Montana specific
Montana State University Extension publications are available online https://store.msuextension.org/ or call 406-994-3273.
Cover Crops as Partial Replacement of Dryland Fallow (MT202111AG)
Cover Crops: Soil Water and Small Grain Yield and Protein (EB0237)
Cover Crops: Management for Organic Matter and Nitrogen (EB0236)
Montana Cool-Season Pulse Production Guide (EB0210)
The Soil Scoop https://landresources.montana.edu/soilfertility/soilscoop/index.html
Evaluating Soil Quality and Health Soil Fertility for Pulse Crops
Eastern Ag Research Center Dryland Cool and Warm Season Cover Crop Performance Evaluations. 2016 Agricultural Research Update http://agresearch.montana.edu/earc/annualreports.html
Evaluation of Multiple Species for Use as Cover Crops in Dryland Production in Montana. 2017. McVay et al. http://www.sarc.montana.edu/PHP/Library/presentations/
Montana Data on Cover Crops. 2016. A presentation by Kent McVay, Southern Ag. Research Center. http://www.sarc.montana.edu/php/Library/presentations/?id=26
USDA-NRCS Soil Erosion Enhancement Activity – SQL12- Intensive cover cropping in annual crops. Montana Supplement. Provides seeding rates for cover crop species. https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb1242878.pdf
USDA-NRCS Bridger Plant Materials Center Technical Notes https://www.nrcs.usda.gov/wps/portal/nrcs/main/plantmaterials/pmc/west/mtpmc/
MT-96. Peas: An Introduced Legume for Conservation Use in Montana and Wyoming. 2014. Hybner, R. 6p. (ID# 12101)
MT-106. Radish: An Introduced Cover Crop for Use in Montana and Wyoming. 2015. Hybner, R. 6p. (ID# 12456)
MT-120. Small-seeded Fava Bean as a Cash Crop and within Cover Crop Mixture. 2017. Tallman, S. 2p. (ID# 13262)
Online tools
Cover Crop Chart. USDA-ARS Northern Great Plains Research Lab. An online or pdf version decision aid tool with information on 58 crop species https://www.ars.usda.gov/plains-area/mandan-nd/ngprl/docs/cover-crop-chart/
Cover Crops for Vegetable Growers. Cornell University. http://covercrops.cals.cornell.edu/decision-tool.php
Crop Sequence Calculator. USDA-ARS, interactive software program to design crop sequences. https://www.ars.usda.gov/plains-area/mandan-nd/ngprl/docs/crop-sequence-calculator/
Organizations
Soil Health Institute https://soilhealthinstitute.org/
Sustainable Agriculture Research & Education (SARE) https://www.sare.org/Learning-Center/Topic-Rooms/Cover-Crops
USDA NRCS MT environmental quality incentives program (EQIP) for cover crop incentives and advice. https://www.nrcs.usda.gov/wps/portal/nrcs/mt/programs/financial/eqip/
Terms Used
Acidification: a decrease in soil pH
Arbuscular mycorrhizal (AM) fungi colonize the host plant’s root tissue, can protect host from pathogens, and increase the host plant’s water and nutrient uptake, especially P.
Available N: N in nitrate (NO3-) and ammonium (NH4+), the two forms of N plants can take up
Bacteria: single cell organisms that break down organic material in the soil
C mineralization: microbial and enzyme activity that breaks down organic material into nutrients, carbon dioxide (CO2), and water
Enzymes: a by-product of organic material breakdown by microbial life in the soil. They are left stuck to clay particles as a “fingerprint.”
Microbial biomass: the total mass/bodies of microorganisms
Microbial biomass C: the carbon in the bodies of microorganisms
Microbial biomass N: the N in the bodies of microorganisms
N Mineralization: microbial and enzyme activity that converts N in organic material into ammonium (NH4+), a form of N that plants can use
Non-AM fungi help break down organic material in the soil. They are not connected to plant roots.
PMN (potentially mineralizable nitrogen) is N in organic material in the soil that can become plant available by microbial breakdown within a growing season.
Soil aggregate stability: the ability of soil clusters to resist breaking down into silt or dust when exposed to water or wind
Soil health is dynamic and characterized by properties such as tilth (physical suitability for planting), microbial activity, N supplying power, and aggregation.
Soil microorganisms: microscopic organisms in the soil which include bacteria, fungi, algae, protozoa, nematodes, and more
Soil organic C: the amount of C in organic material. It is used as in indicator of soil organic matter.
Soil quality includes the properties such as texture and cation exchange capacity, that don’t change easily.
Total soil N: the sum of nitrate (NO3-), nitrite (NO2-), N in organic matter, ammonium (NH4+) and ammonia (NH3-) in the soil

