Biology, Ecology and Management of Montana Knapweeds
This publication provides information on identification, biology and management of spotted knapweed (Centaurea stoebe), diffuse knapweed (Centaurea diffusa), and Russian knapweed (Rhaponticum repens), a group of closely related noxious weeds that have invaded Montana. These weeds are well adapted to a wide range of habitats including open forests, rangeland, roadsides, Conservation Reserve Program (CRP) lands, pastureland and ditch banks. Russian knapweed can also infest cultivated crop and hay land. The knapweeds threaten long-term productivity of Montana grazing lands and wildlands. These aggressive weeds displace native species, change plant community structure, degrade or eliminate wildlife habitat, and reduce forage for livestock. The economic impact to agriculture and wildlands from these weeds is substantial. Successful management of knapweeds requires the use of integrated weed management strategies.
Last Updated: 01/17by Celestine Duncan, Consultant, Weed Management Services, Helena, MT; Jim Story, Research Professor, retired, MSU Western Ag Research Center, Corvallis, MT; and Roger Sheley, former MSU Extension Weed Specialist, Bozeman, MT. Revised by Hilary Parkinson, former MSU Research Associate, and Jane Mangold, MSU Extension Invasive Plant Specialist
PLANT BIOLOGY
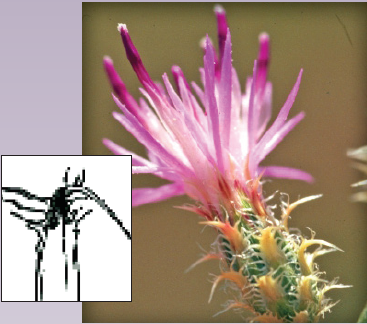
Spotted knapweed (Centaurea stoebe, formerly C. maculosa*) is a tap- rooted, rosette-forming perennial forb that spreads by seed. Stem height varies from 8 to 50 inches (0.2-1.2 m). The slender stems are many- branched and have a single flower at the tip of each branch. Flower color is usually pinkish-purple, but can also be light purple or white (Figure 1A). Flower heads are surrounded by small leaf-like structures called bracts (Figure 1B). The bracts are marked with fine vertical streaks and tipped with a dark comb-like fringe. These bracts give a “spotted” appearance to the flower head.
Rosette leaves are indented or divided about halfway to the midrib (Figure 1C). Rosettes initiate growth in mid-spring, plants typically bolt in early summer and bloom mid-summer to early fall. Individual flower heads bloom for two to six days before the bracts close. Bracts reopen after about 20 days, allowing seed dispersal.
* Latin names follow those currently accepted by the Integrated Taxonomic Information System (ITIS).
FIGURE 1. A) Spotted knapweed flower, B) bract, and C) rosette (photo A by John Cardina, Ohio State University, bugwood.org; B and C by Steve Dewey).
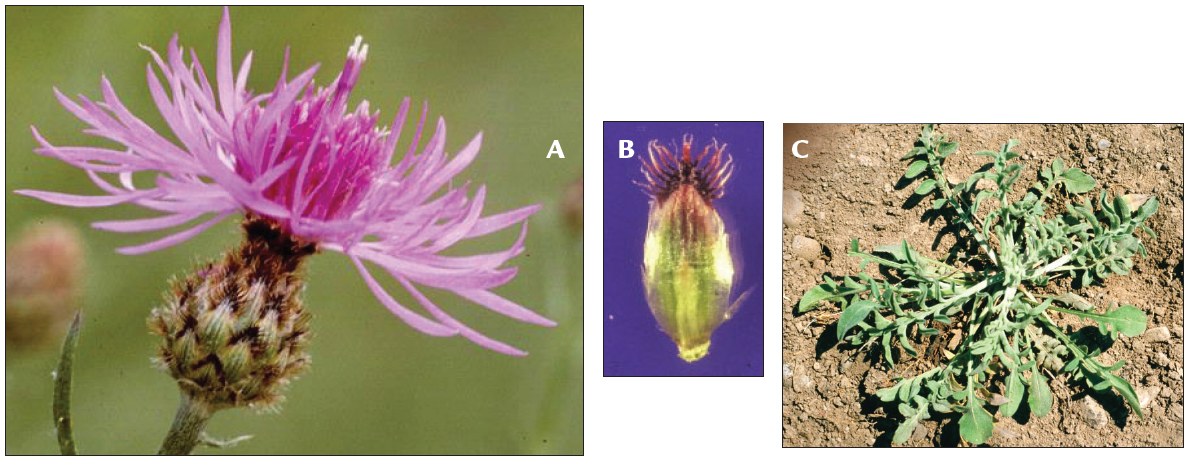
FIGURE 2. Diffuse knapweed rosette (photo by Richard Old, XID Services, bugwood.org), and bract, inset (photo by Steve Dewey).

FIGURE 3. Russian knapweed rosette (photo by Joseph DiTomaso, UC Davis, bugwood.org), and bract, inset (photo by Steve Dewey).

Diffuse knapweed (Centaurea diffusa) is a tap- rooted annual, biennial or short-lived perennial forb that reproduces by seed. Diffuse knapweed typically grows 6 to 24 inches (15-61 cm) tall and consists of a single main stem divided into numerous branches about halfway up the stem, giving it a ball-shaped appearance and tumbleweed mobility. Each branch produces a single flower head. Flowers are usually white (cover, top left), but occasionally light purple. Bracts on diffuse knapweed have a rigid terminal spine about 1/4 to 1/3 inch long with four to five pairs of shorter lateral spines (Figure 2, inset). Bracts can have dark-colored tips but lack the dark fringe present on spotted knapweed. Plants form rosettes (Figure 2) that resemble spotted knapweed. Bolting occurs in early summer, and plants bloom from mid-summer to early fall.
Russian knapweed (Acroptilon repens) is a rhizomatous, deep-rooted, long-lived perennial forb that grows about two feet tall (0.6 m). Stems are thin, stiff and covered with soft, short hairs. Like spotted knapweed, flower color is light pink to purple (cover, bottom left), but two characteristics distinguish Russian knapweed from spotted knapweed and other similarly colored knapweeds: (1) flower head bracts of Russian knapweed have light thin hairs, a papery, translucent tip and are green at the base (Figure 3, inset) and (2) Russian knapweed is rhizomatous instead of tap-rooted. Rosette leaves are narrow at the base and widen toward the tip (Figure 3). Shoots emerge in the early spring, and plants bolt in early summer and flower from mid-summer to early fall.
ECOLOGY
Habitat
Spotted knapweed tolerates a wide range of annual precipitation levels (8 to 80 inches [20.3–203 cm]). It prefers light textured, well- drained soils, but occurs in a wide range of soil types. In Montana it grows in open forests (especially after logging or other disturbances), shrub and grasslands. While it invades disturbed areas more quickly, it can invade healthy, undisturbed plant communities as well. Success increases with disturbance and soil moisture stress. Steep, south facing shrub and grasslands are especially vulnerable. It is common on roadsides, range and pastureland (Figure 4) and ditch banks. Spotted knapweed does not do well in saturated soils such as irrigated pastures and can be outcompeted by healthy grasses at more moist sites.
Diffuse knapweed has similar climatic and soil type preferences to spotted knapweed and is adapted to a wide range of habitats including open forests, range and pastureland, roadsides and ditch banks.
Russian knapweed may occur in the habitats described above, but its tolerance for poorly drained, saline, alkaline soils extends its range to irrigation ditches, flood plains and river corridors. It is likely to be a pest in crops and hay fields where its rhizomatous growth makes it difficult to control. In the north-central part of Montana, it is common in alfalfa and grain fields in the Missouri River bottomlands.
FIGURE 4. Stand of spotted knapweed near Missoula, Montana (Norman E. Rees, USDA Agricultural Research Service, bugwood.org).

Speedy Weed ID
Spotted, diffuse and Russian knapweeds may be confused with each other as well as other exotic knapweeds that occur in Montana in limited numbers and that are not currently state-listed noxious weeds. Following are brief descriptions of other knapweeds that resemble the state-listed species. Differences in bract texture, color or shape aid in identification.
- Brown knapweed (Centaurea jacea,) bracts closely resemble those of Russian knapweed. Both species have a papery translucent tip and no spines or fringes. However, brown knapweed bracts are brown, compared to the yellow-green bracts of Russian knapweed, and brown knapweed is not rhizomatous.
- Squarrose knapweed (Centaurea virgata) bracts closely resemble diffuse knapweed, except squarrose bracts are bent outward compared to diffuse bracts that point upward.
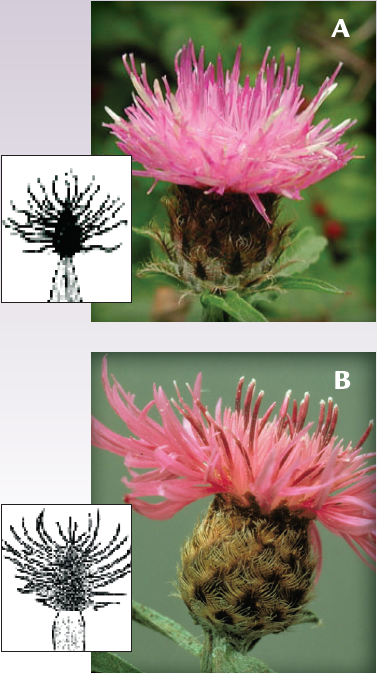
- Black (Centaurea nigra, A) and meadow knapweed (C. debauxii, B) may resemble spotted knapweed, but their fringes are longer than the bracts are wide in contrast to spotted knapweed fringes which are shorter than the bract width. The fringe on black knapweed is black; the fringe on meadow knapweed is tan to brown. The scientific name for meadow knapweed has changed many times. It may also be referred to as C. pratensis, C. nigrescens, C. x moncktonii, C. debeauxii ssp. thuillieri, and C. jacea x nigra.
Descriptions adapted from Roche and Roche, 1993. Knapweed photos: brown by Lee Michels; squarrose by Steve Dewey; black by Eleanor Sulys; meadow by Cindy Roche.
Illustrations courtesy of Cindy Roche.
Spread and Establishment Potential
Spotted knapweed seed production varies from 500 to 4,000 seeds per plant depending on environmental conditions. Seed longevity is greater than eight years. While seeds have no specialized appendages for dispersal, other vectors enable widespread dispersal. Seed heads are caught in the undercarriage of vehicles enabling long distance dispersal. Contaminated crop seed, hay, gravel and road fill also contribute to spread. Wildlife and domestic livestock that consume mature seed heads excrete viable seed seven to 10 days after consumption, providing seed dispersal into remote areas. Seed can be spread via rivers and other waterways, especially when spotted knapweed grows along banks.
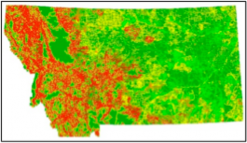
Surveys along major Montana highways (Figure 5) found spotted knapweed is much more abundant in the western part of the state and declines eastward. Presence is also predicted to decline with distance from roads.
Diffuse knapweed seed production may range from 900 to 1200 seeds per plant, but can increase to over 18,000 on irrigated sites. Seed longevity is estimated to be greater than eight years. Seeds buried 1.2 inches (3 cm) or deeper do not germinate. A flush of seedlings may follow a disturbance that brings seeds to the surface. Seeds are spread by the same mechanisms described for spotted knapweed. Additionally, mature plants break at ground level and tumble in the wind spreading seed.
Russian knapweed seed production is highly variable, but generally lower than the other knapweeds. Plants along roadsides or on rangelands average 100 to 300 seeds per plant, but may increase to 1,200 under optimal conditions. Seed longevity is two to nine years. Russian knapweed has no appendages for dispersal, and seed is spread by the same mechanisms as for spotted knapweed. Once established, patches expand mainly by rhizomatous growth. Mature plants can spread radially from established plants’ rhizomes and can cover up to 23 feet (7 m) over two growing seasons. Rhizome fragments created after plowing or other disturbances can also increase spread. Competitive ability and spread is highly dependent on the surrounding plant community. Rhizomatous grasses can suppress this plant, but if competing vegetation is sparse or highly disturbed, or droughty conditions prevail, Russian knapweed is highly competitive.
FIGURE 5. “Risk” map for spotted knapweed occurrence. Red signifies high environmental suitability with declining suitability through orange, yellow, light green and dark green. This map was generated from presence and absence data collected along one mile segments for Montana Department of Transportation maintained highways and interstates during summers of 2003 and 2005. Data were modeled with general linear regression with an addition dispersal function away from roads (Repath et al. 2007).
Damage Potential
Knapweeds are associated with reductions in native plants, reduced forage yields and degraded habitats in range, grasslands and agricultural areas. Based on estimates from 1996, knapweeds cost Montana $42 million per year in direct and indirect costs.
Russian knapweed can cause “chewing disease” in horses, a neurologic disorder that causes brain lesions and mouth ulcers. Symptoms of chewing disease include drowsiness, difficulty in eating and drinking, twitching of the lips, tongue flicking and involuntary chewing movements. There is no cure and horses die of dehydration or starvation. Horses will select other forage when available.
ORIGINS, CURRENT STATUS AND DISTRIBUTION
Spotted knapweed is native to grassland steppes of central Europe and east to central Russia, Caucasia and western Siberia. Spotted knapweed was introduced to North America from Eurasia as a contaminant in alfalfa. It was also introduced through discarded soil used as ship ballast. Spotted knapweed was first recorded in the Northwest in Victoria, British Columbia, in 1883 and in Montana in Ravalli County in 1920. By 1991 the weed had been recorded in every Montana county. Spotted knapweed is the most widespread knapweed in the state, infesting from two to five million acres. Spotted, diffuse and Russian knapweeds are listed as Priority 2B noxious weeds in Montana.
Diffuse knapweed is native to the grassland steppes of eastern Europe and Asia Minor. It grows in the eastern Mediterranean area, in western Asia, and from southern Russia to western Germany. It was first recorded in North America in Washington in 1907 and in Montana’s Mineral County in 1951. Presently, 42 Montana counties have reported the presence of diffuse knapweed (Figure 6). According acreage estimates in the 2017 Montana Noxious Weed Management Plan, diffuse knapweed accounts for less than 1 percent of the total area infested by knapweeds in Montana.
FIGURE 6. Counties in Montana where diffuse knapweed has been reported. (EDDMapS. 2022. Early Detection & Distribution Mapping System. The University of Georgia – Center for Invasive Species and Ecosystem Health. Available online at www.eddmaps.org/; last accessed July 25, 2022.)
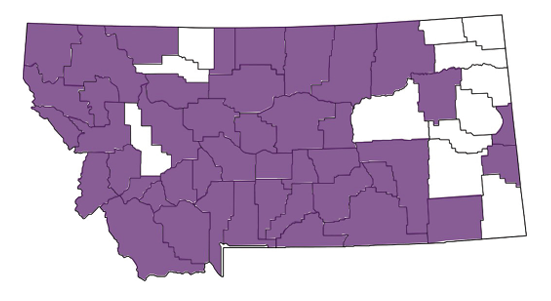
Russian knapweed is native to Mongolia, Russian Turkestan, Iran, Turkish Armenia and Asia Minor. Seeds of Russian knapweed were present in alfalfa seed imported from Russian Turkestan beginning in 1898. Once imported, it spread widely by sale of domestically produced alfalfa seed or hay containing weed seeds. It was first reported in the Northwest in Yakima County, Washington, in 1922 and in Fergus County, Montana, in 1934. By 1991 the weed was recorded in every Montana county and infests an estimated 51,000 acres.
MANAGEMENT ALTERNATIVES
Prevention
Prevention, early detection and rapid response are the keys to preventing the spread of knapweeds onto non-infested range and pasture lands.
Human activities are believed to be one of the largest contributors to the spread of knapweed. Vehicles have been shown to pick up seeds when driven on unpaved roads and off-road even under dry conditions. Such seeds have been recorded to travel over 160 miles under dry conditions with seed dropping off much more quickly under wet conditions. Therefore, a vehicle driven through a spotted knapweed patch has the potential to spread seed hundreds of miles. Avoid driving through infestations, especially when flowering and shortly thereafter and wash the undercarriage of vehicles that have been in weed-infested areas. Construction equipment, fill dirt and gravel (Figure 7) are common sources of weed seed. Where construction, road building, maintenance or some other major disturbance is planned, monitor regularly for several years (bimonthly the first year and monthly for the following three to four years). Do not drive, walk or trail livestock through weed-infested areas. Purchase only certified weed-seed free seed and hay.
FIGURE 7. Knapweed seed can be transported by movement of gravel and other fill material (photo by Jane Mangold, MSU).

Proper grazing management is essential to maintain competitive desired plants, which slow knapweed encroachment. To minimize weed invasion, grazing systems should alter the season of use, rotate or combine livestock types and pastures to allow grazed plants to recover before being regrazed, and promote litter accumulation. On severely degraded, knapweed-infested rangelands, herbicide treatments should be combined with revegetation and rested for two to three years to allow seeded species to establish.
Mechanical Control
Hand pulling: Persistent and careful hand pulling can control spotted and diffuse knapweed. Since regrowth can occur from crowns, the entire crown portion of the plant must be removed. Plants can be pulled most effectively when the soil is moist. If plants are flowering they must be bagged and disposed of in a manner to prevent seed dispersal. While this control method is effective on single plants or relatively small infestations, it is not economically or physically feasible on large, well- established knapweed infestations. Hand pulling is not an effective treatment for established Russian knapweed patches because the plant rapidly resprouts from rhizomes.
Mowing: There has been limited research and mixed results on long-term effects of mowing knapweeds. Mowing can prevent seed production and reduce carbohydrate reserves. It is typically most effective where the surrounding plant community contains healthy perennial grasses that will respond to mowing with renewed growth. By contrast, if the existing plant community is dominated by forbs or annual grasses, mowing may simply open the plant community, reducing competition from surrounding plants, thereby increasing knapweed density.
For spotted knapweed, a Montana study found fall mowing in the flowering or seed producing stage for three consecutive years reduced adult plant density by about 85 percent at two locations. However, mowing is not recommended where biocontrols are well established and serve as the primary control strategy. Mowing at the recommended time (flowering or seed producing stage) will destroy insect larvae deposited in flower heads.
In some cases, herbicide efficacy at low application rates may increase when combined with mowing. For example, mowing at the late bud stage followed by an application of clopyralid + 2,4-D (Curtail® [1 quart per acre]) to fall regrowth provided better control than the herbicides applied alone.
Cultivation: Cultivation to depths of 7 inches (18 cm) or more will control spotted and diffuse knapweed. However, even under intensive cultivation, weeds will regenerate from seeds remaining in soil. Cultivation will increase rate of spread and establishment of Russian knapweed since root sections broken during cultivation will form new plants. Cultivation, in combination with reseeding competitive perennial grasses, may minimize reinvasion of the knapweeds.
Burning: A single, low-intensity fire does not control spotted, diffuse or Russian knapweeds and instead may increase cover and density. Additionally, it creates open areas which facilitate establishment and spread of the knapweeds. However, a controlled burn followed by herbicide may increase effectiveness of herbicide by stimulating and exposing new knapweed growth prior to herbicide application.
Cultural Control
In areas where desirable plant species are absent, long-term control of knapweeds is unlikely without revegetation. Successful establishment of seeded species has been shown to inhibit reinvasion of knapweeds. For example, at sites in western Montana where intermediate wheatgrass (Thinopyrum intermedium) was seeded 15 years earlier, spotted knapweed biomass was reduced by 93 percent. Successful establishment took time and may have been aided by the presence of well-established biocontrol agents.
For dense infestations, herbicide applications are usually necessary to reduce competition, providing time for seeded species to establish. Common approaches include a spring or early summer herbicide application followed by a dormant seeding in late fall; or a fall herbicide application and spring seeding. If the site can be cultivated, another option is to till and seed grasses in the late fall. Both grass and knapweed seedlings will emerge the following spring as long as there is adequate moisture. Knapweed seedlings can be controlled with reduced rates of 2,4-D, clopyralid (Transline®), triclopyr + clopyralid (Redeem®) or clopyralid + 2,4-D (Curtail®) once seeded grasses have established. These broad-leaf herbicides would not be appropriate if forbs or shrubs are included in the seed mix. Revegetation Guidelines: Considering Invasive and Noxious Weeds (see link under Additional Resources) provides more information on implementing
a revegetation program.
For Russian knapweed, a single herbicide treatment followed by reseeding with rhizomatous grasses can provide long term control and avoid annual reapplication of herbicide. In Wyoming, clopyralid + 2,4-D followed by seeding with streambank wheatgrass (Elymus lanceolatus) controlled 66 to 93 percent of the knapweed two years after treatment. By contrast, herbicide alone provided only 7 percent control two years after application, and glyphosate (Roundup®) applied alone increased Russian knapweed growth compared to no herbicide. In another study, a single application of clopyralid + 2,4-D followed by seeding western wheatgrass (Pascopyrum smithii) effectively controlled Russian knapweed.
Biological Control
Insects -
Spotted and diffuse knapweed: Thirteen insects have been introduced into Montana for biological control of spotted and diffuse knapweed (Table 1, page 12). Most of the insects attack both plant species, but some have a preferred host. Insects that have been particularly effective on spotted knapweed in Montana include the root feeding weevil (Cyphocleonus achates), two seed-head feeding weevils (Larinus minutus and L. obtusus), and the seed-head feeding fly (Urophora affinis). Reports released in 2010 found C. achates can reduce plant densities by 77 to 99 percent. The root weevil in combination with the seed head insects has reduced seed production by 94 percent in some places. The root moth (Agapeta zoegana) has also contributed to the reduction of spotted knapweed in several locations in Montana.
For diffuse knapweed, the root beetle (Sphenoptera jugoslavica) and the flower weevil (L. minutus) have done particularly well, especially near Helena, Montana. Larinus minutus has two sites of attack: larvae feed on the seeds, and adults damage leaves of bolting plants. In Colorado grassland where numerous biocontrols were released, L. minutus appeared responsible for most of the reductions in diffuse knapweed.
TABLE 1. Status of insects released in Montana for spotted, diffuse and Russian knapweed.
| Scientific Name | Insect Type | Plant Part Attacked | Date Released | Status |
| Spotted and diffuse knapweed(s) feeds primarily on spotted, (d) feeds primarily on diffuse | ||||
| Agapeta zoegana (s) | Moth | Root | 1984 | Established1 |
| Bangasternus fausti | Weevil | Flower head | 1992 | Unknown |
| Chaetorellia acrolophi (s) | Fly | Flower head | 1992 | Established2 |
| Cyphocleonus achates (s) | Weevil | Root | 1988 | Established1 |
| Larinus minutus (d) | Weevil | Flower head | 1991 | Established1 |
| Larinus obtusus (s) | Weevil | Flower head | 1992 | Established2 |
| Metzneria paucipunctella (s) | Moth | Flower head | 1980 | Established2 |
| Pelochrista medullana (d) | Moth | Root | 1984 | Established3 |
| Pterolonche inspersa | Moth | Root | 1988 | Established3 |
| Sphenoptera jugoslavica (d) | Beetle | Root | 1983 | Established2 |
| Terellia virens (s) | Fly | Flower head | 1992 | Established3 |
| Urophora affinis | Fly | Flower head | 1973 | Established1 |
| Urophora quadrifasciata | Fly | Flower head | 1980 | Established1 |
| Russian knapweed | ||||
| Aulacidea acroptilonica | Wasp | Stem | 2009 | Established2 |
| Jaapiella ivannikovi | Fly | Stem | 2009 | Established2 |
| Mesoanguinea picridis | Nematode | Root | 1990 | Unknown |
Other insects listed for spotted and diffuse knapweed in Table 1 generally have not proven as effective. Before releasing any biological controls, investigate environmental conditions at the site (particularly climate) and select biocontrols that will do well in those conditions. For information on preferred habitats of
biocontrol agents, see the Montana Biological Weed Control Coordination Project (mtbiocontrol.org).
Russian knapweed: Three insects have been released in Montana for control of Russian knapweed. The gall wasp Aulacidea acroptilonica has established well, and its impact is variable depending on year and location. The gall midge Jaapiella ivannikovi can reduce or eliminate seed production at some locations,
however it generally does not build up high populations in Montana and does not establish well at dry sites. The nematode Mesoanguina picridis has failed to establish in Montana. For additional information, see the Montana Biological Weed Control Coordination Project (mtbiocontrol.org).
Grazing -
Cattle, sheep and goats will graze spotted knapweed at low to moderate levels. Although rosettes of first year knapweed plants are nutritious and edible, they are typically not grazed by cattle. Mature spotted knapweed plants are fibrous and coarse, which make them less desirable. Cow-calf pairs have been trained to eat spotted knapweed with some success. Under a short-duration grazing strategy with cattle in western Montana, spotted knapweed seedlings and rosettes decreased, but bare ground increased and litter decreased. Any procedures that increase bare ground on rangeland are not recommended unless integrated with revegetation.
FIGURE 8. A) Sheep grazing knapweed, background, in contrast to ungrazed spotted knapweed, foreground, B) Lamb consuming spotted knapweed stems (photo A by Montana Sheep Institute, B by Gary Mathews).


Sheep grazing can be used to reduce spotted knapweed seed production (Figure 8). If grazing occurs at the bolting stage an additional grazing period is recommended, but if grazing occurs at the late-bud to early flower or full-flower stage, a single grazing period can reduce viable seed production by nearly 100 percent based on studies in Montana. Animals that have grazed knapweed beyond the bolting stage need to be quarantined for seven to 10 days before moving to non-infested pastures in order to prevent seed transport in animal waste.
Combining herbicides with sheep grazing can also effectively control some knapweeds. The herbicides can be used to control mature, unpalatable plants and sheep will selectively remove knapweed rosettes as they re-emerge. Research in Montana found that spring application of 2,4-D followed by grazing with sheep was better than either treatment alone at reducing spotted knapweed cover and biomass. Goats are also recommended to reduce seed production.
Chemical Control
Selective herbicides provide good control of the knapweeds and are often the most cost-effective treatment for small or new infestations (Table 2). In cases where desirable remnant vegetation still exists, persistent spot spraying over two to three years may be enough to release the desirable plants from competition (Figure 9). For denser infestations where desirable vegetation is nearly absent, herbicide treatments are most effective when combined with revegetation or other strategies to enhance the competitive ability of desirable forage species.
Spotted and diffuse knapweed: Each herbicide has special characteristics that make it useful in specific situations. Always consult and carefully read labels before applying herbicides. Picloram provides from two to seven years of control depending on site conditions and is one of the most cost-effective herbicides. In a Montana study, picloram provided better long term control of spotted knapweed and increased grass biomass compared to clopyralid. However, clopyralid applied at the bolting stage was as effective as picloram at one of two sites and provided 50 percent reduction in density at the second site. Due to the long soil residual time of picloram, clopyralid may be a better alternative for more sensitive areas, especially those with higher forb diversity.
Aminopyralid has been highly effective on knapweeds. It has been shown to be as effective as picloram, and the lower use rates pose less risk to the environment. Like clopyralid, it has fewer impacts on non- target plants. In field trials at 10 locations spanning four states, only 14 of 68 desired forb species were moderately susceptible or susceptible to aminopyralid applications at 5 to 7 oz/acre.
A limited number of studies have investigated the compatibility of herbicides with biocontrols and results are mixed. For the root feeding A. zoegana and C. achates, late spring applications of 2,4-D, or clopyralid were preferred over fall applications which can substantially reduce larval numbers. However, the number of spotted knapweed seed heads impacted by Urophora species was not different between plots sprayed with picloram or 2,4-D compared to non-sprayed plots. In general, herbicide applications are not recommended in the first couple of years following release of biocontrols.
Russian knapweed: Rhizomatous growth of Russian knapweed can make it particularly difficult to control with herbicides. Except for 2,4- D, optimal timing for herbicide application on Russian knapweed is generally later compared to the other knapweeds (early bud to flowering compared to rosettes to bolting stages). Combining herbicides with revegetation is strongly recommended to provide long term control.
TABLE 2. Herbicides, rates and application times for control of knapweeds.
| Spotted or Diffuse | Russian | |
| 2,4-D | 1-2 lb ae/acre. Apply at early stage of bolting. | 4-8 lb ae/acre. Apply at early stage of bolting. |
| Aminopyralid (Milestone®) | 5-7 fl oz/acre. Apply from rosette to the bolting stages or in the fall. | 5-7 fl oz/acre. Apply from early bud to flowering and to dormant plants in the fall. |
| Clopyralid (Transline® or Stinger®) | 1⁄3-1 pint/acre. Apply after majority of basal leaves have emerged up to bud stage or fall regrowth. See label for site specifics. | 0.25-0.5 lb ae/acre (0.66-1.33 pints/acre). Apply up to bud stage. See label for site specifics. |
Clopyralid + 2,4-D (Curtail®) |
2-3 quarts/acre (higher rate for dense patches or poor growing conditions). Apply after rosettes emerge but before bolting. | 3-4 quarts/acre. Apply at the early bud to mid-flowering stage or on fall regrowth. |
| Picloram (Tordon 22K®) | 1-2 pints/acre. Apply from rosette to mid-bolting stage or to fall regrowth. | 2-4 pints/acre. Apply during active growth from bud to mid-flowering or to fall regrowth. |
| Triclopyr + clopyralid (Redeem R&P®) | 1.5-2 pints/acre. Apply from rosette to early flower or to fall regrowth. Optimum time is mid-bolt. | 3- 4 pints/acre. Apply from bud to mid-flower stage or fall regrowth. |


FIGURE 9. Left, dense knapweed along trail in Bear Trap Canyon, Madison County, Montana, in fall 2001. Right, the same site in fall 2003 with abundant grasses and near absence of knapweed. Treatment included spot-spraying in late May and again in late July to early August (when the plants began to flower) with 1 quart/A picloram (Tordon® 22K) + 1 quart/A 2,4-D (photos courtesy of the BLM Dillon Field office who organized and implemented this weed control program).
INTEGRATED WEED MANAGEMENT (IWM)
Successful management of knapweeds requires the use of integrated weed management strategies. This includes combining strategies to prevent the movement of these weeds, containing existing infestations, and integrating control methods to reduce weed infestations to tolerable levels. The goal of a management program should be to develop healthy plant communities that are invasion resistant and meet land-use objectives. Preventing weed seed spread onto adjacent land is the most cost-effective management strategy. The following practices will reduce or eliminate knapweed seed dispersal and establishment:
- Learn to recognize knapweeds and report new occurrences.
- Eradicate small patches of knapweeds before they have a chance to spread.
- Refrain from driving vehicles through infestations and wash vehicles in a designated area before travelling into non-infested areas.
- Purchase and transport only certified noxious, weed-seed free forage.
- Minimize soil disturbance on range and other non-crop lands.
- Use IWM to contain large knapweed infestations.
- Seed desirable perennial grass species on areas disturbed by construction, mining or other activities.
- Support local weed management programs.
ADDITIONAL RESOURCES
Benz L.J., K.G. Beck, T.D. Whitson, and D.W. Koch. 1999. Reclaiming Russian knapweed infested rangeland. Journal of Range Management. 52(4): 351-356.
Benzel, K., T. Mosley, and J. Mosley. 2009. Defoliation timing effects on spotted knapweed seed production and viability. Rangeland Ecology and Management. 62(6): 550-556.
Bottoms, R.M. and T.D. Whitson. 1998. A systems approach for management of Russian knapweed (Centaurea repens). Weed Technology. 12:363-366.
Brown, M., C.A. Duncan and M.B. Halstvedt. 1999. Spotted knapweed management with integrated methods. Proceedings Western Society of Weed Science. 52: 68-70.
Corn, J.G., J.M. Story and L.J. White. 2009. Comparison of larval development and overwintering stages of the spotted knapweed biological control agents Agapeta zoegana (Lepidoptera: Tortricidae) and Cyphocleonus achates (Coleoptera: Curculionidae) in Montana versus eastern Europe. Environmental Entomology. 38(4): 971-976.
Duncan, C.L. and J.K. Clark, eds. Invasive plants of range and wildlands and their environmental, economic, and societal impacts. Weed Science Society of America, 2005.
Orloff, N., M. Pokorny, J. Mangold, J. Marks, B. Christiaens, and S. Rogge. 2022. Revegetation Guidelines: Considering Invasive and Noxious Weeds. Extension Bulletin 0242, https://store.msuextension.org/Products/
Revegetation-Guidelines-Considering-Invasive-and-Noxious-WeedsEB0242__EB0242.aspx
Halstvedt, M., D. Cummings, T. Almquist, L. Samuel, R. Lym, G. Beck, R. Becker, C. Duncan and P. Rice. Fall 2010. Native shrub and forb tolerance to Milestone® herbicide. Techline Newsletter.
Lacey, J.R., C.B. Marlow and J.R. Lane. 1989. Influence of spotted knapweed on runoff and sediment yield. Weed Technology. 3: 627-631.
Laufenberg, S.M., R.L. Sheley, J.S. Jacobs and J. Borkowski. 2005. Herbicide effects on density and biomass of Russian Knapweed (Acroptilon repens) and associated plant species. Weed Technology. 19(1): 62-72.
Lesica, P. 1991. The effect of the introduced weed, Centaurea stoebe, on Arabis fecunda, a threatened Montana endemic. Montana Natural Heritage Program, State Library, Helena, Montana.
Mangold, J.M., C.L. Poulsen and M.F. Carpinelli. 2007. Revegetating Russian knapweed (Acroptilon repens) infestations using morphologically diverse species and seedbed preparation. Rangeland Ecology and Management. 60(4): 378-385.
Panter, K.E. 1991. Neurotoxicity of the knapweeds (Centaurea spp.) in horses. In: Noxious Range Weeds. L.F. James, J.O. Evans, M.H. Ralphs, R.D. Child (eds.). Westview Press, Boulder, Colorado. pp. 316-324.
Perry, L.G., C. Johnson, E.R. Alford, J.M. Vivanco and M.W. Paschke. 2005. Screening of grassland plants for restoration after spotted knapweed invasion. Restoration Ecology. 13(4): 725-735).
Repath, C.F., L.H. Rew and F.L. Dougher. 2007. Inventory and probability of occurrence maps for state listed noxious weed species. Technical Report submitted to Montana Department of Transportation.
Rice, P., M.D. Bendunah and C. Carlson. 1992. Plant community diversity after herbicide control of spotted knapweed. USDA Forest Service Intermountain Research Station Paper INT 460.
Rinella, M., J. Jacobs and R. Sheley. 2001. Spotted knapweed response to season and frequency of mowing. Journal of Range Management. (54): 52-56.
Rinella, M.J., J.M. Mangold, E.K. Espeland, R.L. Sheley and J.S. Jacobs. 2012. Long-term population dynamics of seeded plants in invaded grasslands. Ecological Applications. 22:1320-1329.
Roche, C.T. and B.F Roche. 1993. Identification of knapweeds and starthistles in the Pacific Northwest. Pacific Northwest Extension Bulletin 432. Washington State University Extension.
Seastedt, T.R., N. Gregory and D. Buckner. 2003. Effects of biocontrol insects on diffuse knapweed (Centaurea diffusa) in a Colorado grassland. Weed Science. 51: 237-245.
Sheley, R.L., B.E. Olson and L.L. Larson. 1997. Effect of weed seed rate and grass defoliation level on diffuse knapweed. Journal of Range Management. 50:39-43.
Sheley, R.L., J.S. Jacobs and M.F. Carpinelli. 1998. Distribution, biology, and management of diffuse (Centaurea diffusa) and spotted knapweed (Centaurea maculosa). Weed Tech. 12:353-362.
Sheley, R.L., J.S. Jacobs and J.M. Martin. 2004. Integrating 2,4-D and sheep grazing to rehabilitate spotted knapweed infestations. Journal of Range Management. 57: 371-375.
Sheley, R.L., C.A. Duncan, M.B. Halstvedt and J.S. Jacobs. 1999. Spotted knapweed and grass response to herbicide treatments. Journal of Range Management. 53(2): 176-182.
Stannard, M. 2004. Basic biology, distribution and vegetative suppression of four knapweed species. USDA Natural Resource Conservation Service. Technical Note 18. https://www.nrcs.usda.gov/plantmaterials/
idpmstn4913.pdf
Story, J.M., W.R. Good, L.J. White, and L. Smith. 2000. Effects of the interaction of the biocontrol agent, Agapeta zoegana L. (Lepidoptera: Cochylidae), and grass competition on spotted knapweed. Biological Control. 17: 182-190.
Thompson, M.J. 1996. Winter foraging response of elk to spotted knapweed removal. Northwest Science. Vol. 70(1): 10-19.
Zouhar, K.L. 2001. Acroptilon repens. 2001. Centaurea diffusa. 2001. Centaurea maculosa. (three separate documents). In: Fire Effects Information System [Online]. U.S. Department of Agriculture, Forest
Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). https://www.fs.usda.gov/database/feis/plants/forb/acrrep/all.html
ACKNOWLEDGEMENTS
Publication of this bulletin was made possible with a grant from the Montana Department of Agriculture Noxious Weed Trust Fund, and USDA-National Institute of Food and Agriculture. The authors would like to thank Monica Pokorny and Noelle Orloff for reviewing the revised edition and MSU Extension
Communications for graphic design.