Knotweed Complex (Fallopia and Persicaria spp.)
Japanese knotweed, giant knotweed, Himalayan knotweed, and Bohemian knotweed are perennial, rhizomatous plants resembling bamboo with their hollow stems and rapid, aggressive growth habits. They were introduced to the United States in the late 1800s from Asia, predominantly as ornamentals. Members of the knotweed complex can outcompete existing vegetation to form dense monotypic stands, and they are extremely difficult to control. The most common method of spread is rhizome fragments dispersed along waterways or in transported soil. Control options include repeated cutting or hand pulling and herbicides (by foliar application or stem injection).
Last Updated: 08/17by Hilary Parkinson, Research Associate, MSU Department of Land Resources Sciences; and Jane Mangold, MSU Extension Invasive Plant Specialist, Department of Land Resources and Environmental Sciences
PLANT BIOLOGY
Identification
Species in the knotweed complex are in the family Polygonaceae and are a group of large, rhizomatous, herbaceous perennial plants varying in height from about 5 feet (1.5 m) to more than 19 feet (5.8 m) tall. Species typically included in the complex that are highly invasive in many states are Japanese knotweed (Fallopia japonica, cover); giant knotweed (Fallopia sachalinensis, Figures 1A and B); and Bohemian knotweed, a hybrid between giant and Japanese knotweed (Figures 2A and B, page 4). Bohemian knotweed is the most widespread knotweed in the western U.S. Himalayan knotweed (Persicaria wallichii) is not included in the knotweed complex on the Montana noxious weed list, but it is included in this bulletin.
FIGURE 1. Growth habit (A), and greenish- to creamy-white flowers (B) of giant knotweed. (photos by Linda Wilson)


FIGURE 2. Bohemian knotweed growth habit (A), and leaves (B). (photos by Jennifer Andreas, Washington State University Extension)


Plants in the knotweed complex have two characteristics to distinguish them from most other related native or non-native plants in Montana: (1) alternate leaves grow on hollow, bamboo-like stems that grow in clumps; and (2) the nodes (which are not hollow) have a papery or membranous sheath (Figure 3).
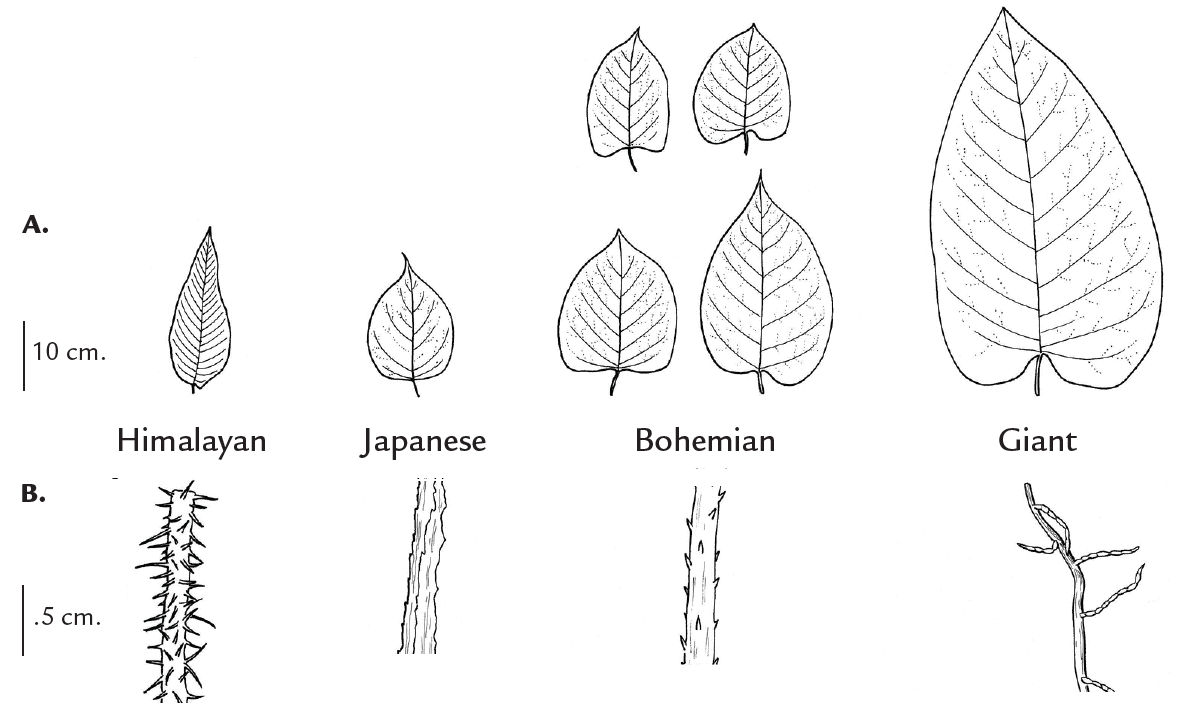
Physical differences among the four species are summarized in Table 1 on page 18. Giant knotweed is the largest and can grow up to 19 feet (6 m) tall. Japanese knotweed tends to be the shortest ranging from five to eight feet (1.5-2.5 m), but it can overlap in height with Himalayan knotweed and Bohemian knotweed. Flowers are greenish-white to creamy-white, except for Himalayan knotweed which has pinkish-white to pink flowers (Figure 2B).
Leaf shape and the surface of veins on the underside of the leaves are vegetative characteristics that can be used to distinguish among knotweed species (Figure 4). The Himalayan knotweed leaf is narrow; its width is less than half of its length, which distinguishes it from the giant, Japanese and Bohemian, which have leaf widths more than 2/3 of their length (Figure 4A). The leaf base of giant knotweed is deeply heart shaped, compared to the base of Japanese knotweed which is flat (forms a right angle with the leaf stem).
FIGURE 3. The membranous sheaths at stem nodes (circled), along with the hollow stems help identify plants in the knotweed complex.

FIGURE 4. Differences in (A) leaf shapes and (B) hairs on the underside leaf veins of Himalayan, Japanese, Bohemian and giant knotweeds. (line drawings by Cindy Roché)
Speedy Weed ID
How do you know if the plant is in the knotweed complex?
If it is one of the four species in the knotweed complex, it should have the following characteristics:
- Erect, hollow, bamboo-like stems with obvious nodes (joints which are not hollow)
- A membranous sheath at each stem node (Figure 3)
- Perennial, rhizomatous growth
- Flowers emerge from where leaves meet the stem
- Flowers with 5 petals (technically tepals), rarely 4, all of which are upright
The leaf shape of Bohemian knotweed (Figure 2B) is variable and may resemble either parent (giant and Japanese knotweed). Bohemian knotweed’s small stout hairs on the underside leaf veins distinguish it from the multi-cellular hairs on giant knotweed veins, and the rough ridges (but absence of hairs) on Japanese knotweed veins (Figure 4B). A magnifying glass is needed to see these differences. If you need help identifying a plant that you believe is in the knotweed complex, contact your local Extension agent or weed coordinator, or send a sample to Montana State University, Schutter Diagnostic Laboratory, 121 Plant BioScience Building, Bozeman, MT 59717-3150.
The plague of escaped ornamentals
While many invasive plant species arrive to a new place accidentally, others, like knotweed, were introduced intentionally as ornamentals. Homeowners valued the feathery white flowers and bamboo- like stems that could rapidly form a privacy screen. Unfortunately, the unsuspecting homeowner did not anticipate that what was planted as a decorative hedge had the potential to form a nearly impenetrable wall (Figures 5 and 6). Nor did they expect it to make its way to nearby streams to form a dense blockade (Figure 9), limiting access for anglers, outcompeting native plants, and altering wildlife habitat. Examples of other escaped ornamentals are purple loosestrife (Lythrum salicaria), Dalmatian and yellow toadflax (Linaria dalmatica, and L. vulgaris), oxeye daisy (Leucanthemum vulgare), and yellowflag iris (Iris pseudacorus). Unfortunately, many of these are still available in nurseries or mail order catalogs. Before purchasing a plant, inquire about the plant’s invasiveness. In many cases, a native plant can be found to fulfill the landscape characteristic desired. For example, elderberry (Sambucus cerulea or S. racemosa) is a mid-sized shrub with clusters of creamy white flowers similar to Japanese knotweed and will thrive in moist conditions. Landscaping with native plants will reduce the risk of invasion, reduce fertilizer and water use, and often provide better habitat for wildlife such as songbirds.
FIGURE 5. Japanese knotweed planted as a hedge, but now dwarfing a parked car in Lincoln County, Montana. (photo by Dan Williams)
FIGURE 6. A hedge of Japanese knotweed that formed a nearly impenetrable wall around the perimeter of a house in Lincoln County, Montana. (photo by Dan Williams)
Life History
Knotweed plants are herbaceous perennials that produce new shoots from rhizomes and crowns. New shoots emerge from mid-spring to late summer. Sprouts are fleshy, pointed at the tip, and slender, resembling asparagus shoots. New shoots may not be hollow until they mature.
Following emergence, growth is rapid: Japanese knotweed can grow two to four inches (5-10.2 cm) per day in the spring. Flowering occurs in late summer, from August to September, with fruit set beginning in September. Knotweed stems and leaves are not frost tolerant, and at the onset of cold temperatures, above ground growth dies; although, dead canes often remain upright and fruits sometimes remain on the stem throughout winter.
In all four species, reproduction is primarily vegetative by rhizome fragments. Himalayan and giant knotweed plants may occasionally produce seed, while seed production in Japanese and Bohemian is rare. This is due to differences in flower types. Himalayan and giant knotweeds bear perfect flowers (with male and female parts in the same flower). Japanese and Bohemian are gynodioecious, meaning flowers may be perfect, or female only. In Japanese knotweed’s escaped range, only the female form is typically present, meaning no seeds are produced due to lack of pollen. Occasionally a mixed-sex population of Japanese knotweed occurs which may regularly produce seed. Bohemian knotweed may more frequently have perfect flowers rather than female flowers. If a giant knotweed plant occurs in the vicinity of a female Japanese or Bohemian knotweed patch, it can provide pollen, allowing seed production. Additionally, fertile pollen from a perfect Bohemian knotweed plant may backcross to fertilize a female Japanese knotweed plant.
When seed is produced, it typically forms two to three weeks after flowering. Seed germinability increases the longer the seed is on the stem. A study in Pennsylvania found Japanese knotweed seed collected in early September had germination rates below 10 percent, while seed collected four weeks later had germination rates as high as 90 percent.
ECOLOGY
Habitat
Japanese knotweed is native to Japan, China, Korea and Taiwan. It is considered an early successional species and is commonly the first plant to colonize volcanic slopes. Giant knotweed is native to northern Japan. It is similar to Japanese knotweed in its ability to colonize disturbed areas. Bohemian knotweed was first described in eastern Europe. It is not clear whether Bohemian knotweed in the Americas came vegetatively from European sources or developed from crosses between giant and Japanese knotweeds on American soils. Himalayan knotweed is native to the Himalayan region of southern Asia. In its native range, it proliferates with disturbance, spreading in avalanche prone areas, along tree lines, and on eroded slopes.
FIGURE 7. Rhizomes of an excavated knotweed plant. (photo by John Cardina, Ohio State University, Bugwood.org)

FIGURE 8. A new plant that generated from a stem fragment. (photo by Dr. Tim Miller, WSU- Northwest Research and Extension Center)

In Europe and the United States, plants in the knotweed complex are often found in yards and managed landscapes where they were intentionally planted. Escaped plants are most commonly found in moist areas such as along riverbanks, canals, wetlands, and lakeshores. However, they can tolerate a range of moisture conditions and also occur frequently in disturbed areas like utility pathways, waste places, strip- mining areas, and along roadways.
Japanese knotweed and giant knotweed are unlikely to invade forests or areas with a closed canopy because they are considered shade intolerant. However, in the Cascades, Japanese knotweed grows in open canopies of black poplar (Populus nigra), red alder (Alnus rubra) and Douglas fir (Pseudotsuga menziesii). More open forest canopies in Montana may be vulnerable. Bohemian and Himalayan knotweed tolerance to shading is unknown but it is likely similar to that of Japanese and giant. Knotweeds are rarely limited by soil types or nutrient levels; research on Japanese knotweed found nutrient-poor sites do not prevent its establishment or survival, but only reduce its growth rate compared to nutrient-rich sites. Japanese knotweed is found in soils with pH ranging from 4.5 to 7.4. Information is limited on the pH ranges for the other species.
FIGURE 9. Dense stands of knotweed reduce diversity, impact habitat, and impede river access for wildlife and anglers. (photo by Leslie J. Mehrhoff, University of Connecticut, IPANE)

FIGURE 10. Japanese knotweed that has expanded beyond the bed it was planted in with rhizomes emerging through concrete walk way. (photo courtesy of Ravalli County Weed District)

Spread and Establishment Potential
The invasiveness of knotweed is primarily due to vigorous rhizomes (Figure 7). Rhizome fragments are spread when soil from a knotweed patch is excavated and moved off site, or when rhizomes from plants growing along a riverbank break off and float downstream. Rhizome fragments as small as 0.02 lb (7 g) can regenerate, provided a node is present. They can also regenerate when buried up to depths of three feet (0.9 m), and they have been observed to emerge through two inches (5 cm) of concrete. The hybrid Bohemian knotweed has been reported to have better regeneration ability than both parents. Stem fragments can also serve as a mode of spread. If a cut stem lands on moist soil or grass, root and shoots may emerge and develop into a new plant (Figure 8).
Once a propagule has landed in a suitable habitat, whether as a rhizome fragment, stem section, or seed (least common), it can grow rapidly. Underground rhizomes can grow 50 to 65 feet (15.2-19.8 m) laterally and produce new shoots, thus plants can establish firmly and expand locally.
Damage Potential
Knotweed can form extensive, monotypic stands, especially in moist areas. It can line the banks of creeks and rivers, forming nearly impenetrable walls (Figure 9). These dense stands are associated with changes in water quality and food chains, and they may impact fisheries. After crowding out native vegetation, knotweed leaves banks vulnerable to erosion when it dies back in winter.
Damages to infrastructure by knotweed rhizomes and stems are well documented (Figure 10). Plants can push through concrete, displacing foundations, walls, pavements, and drainage works. In England, heavy duty barriers with 50 year warranties are required for the foundations of buildings constructed in areas infested with Japanese knotweed. On the east coast of the United States, growth of Japanese knotweed along road- ways reduces sight distances, blocks road signs, and damages pavement.
Differences among species in the knotweed complex regarding their ability to impact ecosystems and infrastructure are not well documented. Most of the research recording the impacts of plants in the knotweed complex has focused on Japanese knotweed, though many were likely Bohemian knotweed misidentified as Japanese knotweed. Reports from Europe suggest that Bohemian knotweed may be more competitive than Japanese or giant knotweed and may spread at a faster rate.
CURRENT STATUS AND DISTRIBUTION
Of the four species in the complex, Japanese knotweed is the most widely reported in North America (Figure 11A). In the Pacific Northwest, Japanese knotweed is most abundant along the coasts of Washington, Oregon, Alaska and British Columbia. The distribution of giant knotweed is similar to Japanese knotweed, except it is not reported in most of the Midwestern states (Figure 11B). Bohemian knotweed has been reported in Idaho, Montana, Oregon, Washington, British Columbia, Ontario, and Quebec (Figure 11C). Recent research suggests that its distribution is much more widespread than reported because Bohemian knotweed is frequently misidentified as Japanese knotweed. Reports of Himalayan knotweed are limited to the west coast except for Montana, Massachusetts and Nova Scotia (Figure 11D).
FIGURE 11. States and provinces with records of plants in the knotweed complex in North America. (NRCS Plants Database, http://plants.usda.gov/. Maps have been modified to reflect additional records not shown on NRCS maps.)
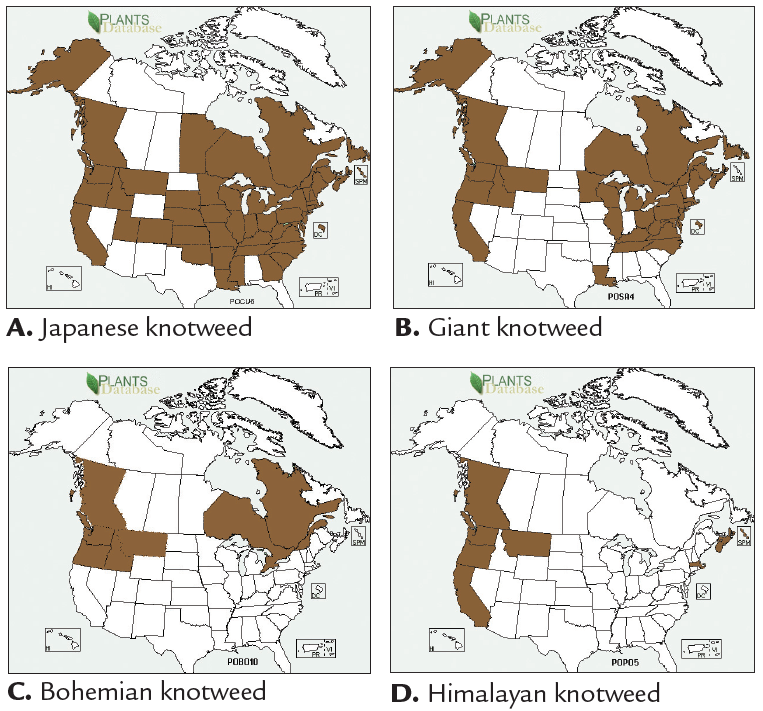
FIGURE 12. Montana counties reporting Japanese (A), giant (B), and Bohemian (C) knotweed. (Compiled records from INVADERS Database System, EDDMapS West, Consortium of Pacific Northwest Herbaria, and MSU Schutter Diagnostic Laboratory.)
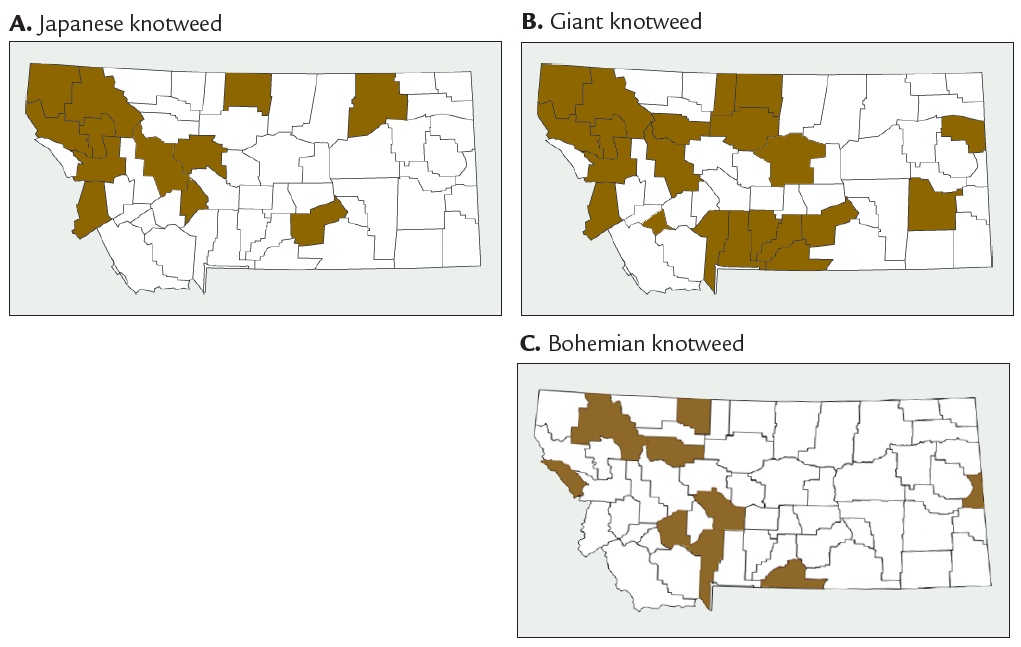
In Montana, Japanese knotweed has been reported in 16 counties (Figure 12A). Giant knotweed has been reported in 21 counties (Figure 12B). Bohemian knotweed has been reported in nine counties (Figure 12C). Himalayan knotweed was reported in Sanders County in 1985. These maps should be interpreted with caution because knotweed species can be difficult to distinguish from each other.
Japanese, giant and Bohemian knotweed are priority 1B noxious weeds in Montana. Such weeds have limited presence in Montana and management criteria for them are eradication or containment and education. Japanese knotweed is also listed as a noxious weed in Alabama, California, Connecticut, Idaho, Massachusetts, New Hampshire, Oregon, Vermont and Washington. Giant knotweed is listed as noxious in California, Colorado, Idaho, Oregon, South Dakota, and Washington. Bohemian knotweed is listed as noxious in Idaho, Washington and Canada. Himalayan knotweed is listed as noxious in California, Oregon and Washington.
MANAGEMENT ALTERNATIVES
Knotweed control efforts will typically require a combination of treatments over multiple years. Anecdotally, Himalayan is considered the easiest to control and Bohemian knotweed the most difficult. Most of the research on control methods has focused on Japanese knotweed. Until more research is available, the recommendations for the other three species are based on recommendations for Japanese knotweed control, except where noted. The area within a 60 foot radius of the original patch should be monitored regularly for several years following treatment, even after the patch appears to be eradicated.
Mechanical control
Stem Cutting, while labor intensive, is effective. Persistent cutting over many years is required and cutting at least three times per year is needed to significantly reduce rhizome reserves. There is some risk that cutting will exacerbate lateral rhizome expansion. For greatest effect, the last cutting should occur before plants begin to lose their leaves with the onset of winter. Where possible, mowing is also effective if repeated for several years. Mower height should be as close to the ground as possible, and mowing should be repeated when plants reach a height of six (15.2 cm) inches. Mowing should continue throughout the growing season until a killing frost occurs.
Knotweed that is cut, hand pulled, or mowed can easily regenerate. Stems and all excavated material should be placed on a dry surface such as a tarp or concrete until dried out and the risk of regeneration is gone, or the material should be burned.
Hand pulling or digging is effective if done consistently on new, small patches and when plants are young and the soil is moist. The patch should be treated twice monthly to remove new sprouts as they emerge.
Tilling alone is not recommended as it can increase new sprouts by breaking up rhizomes into fragments. However, it may be used in combination with other treatments, like mowing re-sprouts or to promote leaf production prior to herbicide application.
Chemical Control
Herbicides labeled for control of knotweed are described in Table 2. Stem injection is one of the more commonly recommended methods. This involves using a hand-operated injection device designed to deliver repeated, pre-measured doses. Prior to injection, a hole must be made using an awl or other pointed tool. All stems must be treated. Plants may also be broadcast sprayed (see formulation with imazapyr or aminopyralid, Table 2).
Foliar applied glyphosate can also be quite effective using rates ranging from 2.5 to 8 percent. While herbicide labels do not recommend a specific application time, reports suggest glyphosate products are most effective from July to September or prior to leaves discoloring and falling off. Fall application may be most effective because plants will translocate more herbicides to rhizomes rather than above-ground growth.
TABLE 2. Examples of herbicides for management of the knotweed complex. The active ingredient is in italics followed by a common trade name. Consult herbicide labels for additional rate, application, restriction, and safety information as well as recommendations for adjuvants or surfactants. Additional herbicide information can be found at http://www.greenbook.net.
|
INGREDIENT TRADE NAME |
MODE OF ACTION | DIRECTIONS |
|
Aminopyralid Milestone |
Synthetic auxin |
Apply as broadcast spray at 7 to 14 oz/acre when plants are 3 to 4 feet tall. |
| Glyphosate | Inhibition of EPSP synthase |
Inject 0.17 oz (5 mL) into the hollow stem between the second and third node, or approximately 6" (15.2 cm) above the ground. |
| Glyphosate | ||
|
Campaign |
Inhibition of EPSP synthase; action like indole acetic acid (synthetic auxin) | Inject 0.2 oz (6 mL) into the hollow stem between the second and third node. |
|
Lineage Clearstand |
Inhibition of acetolactate synthase |
Apply as broadcast spray at 25 oz/acre for total vegetation control (non-selective). Apply postemergence at any time during growing season. |
|
Arsenal |
Inhibition of acetolactate synthase |
Apply 4-6 pints/acre using the higher rate for dense, well-established patches. Apply when plants are actively growing and include a surfactant. For non-crop areas only. |
|
Habitat |
Inhibition of acetolactate synthase |
For application in and around water.Apply 3 to 4 pints/acre postemergenceto actively growing foliage. Use an adjuvant that is approved for aquatic use. |
Cultural Control
Revegetation alone is unlikely to be an effective control method. Other measures should be taken to first control knotweed (combination of digging/grubbing, mowing and herbicides). Once a patch appears to be eradicated, revegetation is strongly recommended to suppress reinvasion. The following species are recommended for revegetation: shrubs such as willow (Salix spp.), American elderberry (Sambucus canadensis, S. cerulea or S. racemosa), or alder (Alnus serrulata and A. incana ssp. rugosa); grasses such as streambank wheatgrass (Elymus lanceolatus) or Great Basin wildrye (Leymus cinereus). Species should be selected based on conditions of the site.
Covering plants with heavy black plastic or cardboard for more than one year may suppress the plant. This is recommended for very small infestations. Rhizomes may remain dormant for up to 20 years, so the lack of regrowth in years following removal of the covering does not mean the plant is dead, and regular monitoring is required.
Biological Control
Grazing has been observed to reduce the establishment and growth of Japanese knotweed where grazing pressure is high. Young shoots are palatable to sheep, goats, cattle and horses. Goats may be most effective, but more research is needed. Grazing will not kill the plants, but repeated grazing can weaken them.
Insects and Pathogens: At this time no insects have been approved for biological control, but there are some promising candidates. These include a leaf chewing beetle (Gallerucida bifasciata), a rust pathogen (Puccinia polygoni-amphibii var. tovariae), a plant-feeding insect (Aphalara itador, a type of psyllid) and a leaf-spot fungus (a Mycosphaerella species). More research on host range and specificity is needed before any can be approved for release.
INTEGRATED WEED MANAGEMENT (IWM)
Prevention of knotweed establishment must be the number one priority for management, because once established, eradication is extremely difficult. While plants in the knotweed complex occur in scattered areas across the state, they do not yet line rivers or roadways like in other regions. Preventing further spread can be achieved by identifying and suppressing existing patches. Do not spread soil from an area with knotweed to other areas, because this soil will contain root fragments that can easily regenerate into new plants.
For existing patches, an integrated management strategy is recommended. Cutting the stems one to three times during the growing season prior to mid-summer and then applying herbicide in late summer or early fall is effective. This can increase herbicide efficacy by stimulating new growth which may be more susceptible to herbicide activity. Additionally, clipping will reduce the amount and height of foliage to be sprayed, making the patch more accessible. Other mechanical treatments like grazing or mowing may be substituted for clipping (up to mid- summer) in combination with a fall herbicide application.
Patches should be monitored for many years after re-growth appears to have ceased. Herbicide applications and other mechanical treatments will leave the area exposed to other invasive weeds. After patches appear to be successfully controlled, the site should be revegetated with appropriate species if desirable vegetation is not returning naturally. See revegetation in the cultural section above for species recommendations, or consult your local Extension or NRCS office.
GLOSSARY
Alternate - leaf arrangements with only one leaf per node. This is in contrast to an opposite leaf arrangement where two leaves arise per node, in opposed pairs, one on each side of the stem.
Crown - persistent base of a perennial plant.
Gynodioecious - having both male and female parts on some plants, and only female parts on other plants of the same species.
Herbaceous - plant that has leaves and stems that die down at the end of the growing season to the soil level, or a plant lacking a permanent woody stem.
Nodes - the parts of a plant stem from which one or more leaves emerge, often forming a slight swelling or knob.
Perfect - a plant containing both male and female parts. The pollen is produced in the same flower that produces the seed. A perfect flower can pollinate itself and does not need wind, insects or other means to transfer its pollen.
Propagule - any plant part with the capacity to give rise to a new plant. For example, a seed or a vegetative part that is capable of independent growth if detached from the parent plant (such as the stem or root fragments in the case of knotweeds).
Rhizomatous - root growth from rhizomes.
Rhizome - a root-like subterranean stem commonly horizontal in position that usually produces roots below and sends up shoots progressively from the upper surface.
Tepals - flowers typically contain an inner arrangement of petals surrounded by an outer arrangement of sepals, or petal-like structures that are green or greenish and leafy in texture. Knotweeds do not have sepals. When flowers do not have a set of both petals and sepals, the petals are called tepals.
ADDITIONAL RESOURCES
Wilson, L.M. 2007. Key to identification of invasive knotweeds in British Columbia. B.C. Ministries of Forest and Range, Forest Practices Branch, Kamloops, British Columbia, http://www.for.gov.bc.ca/hfp/biocontrol/downloads/Knotweed_key_BC_2007.pdf
REFERENCES
Andros, C.F. 2007. Japanese knotweed becoming an important fall honey plant in the northeast. American Bee Journal, 147(5):372-373.
Barney, J.N., N. Tharayil, A. DiTommaso, and P.C. Bhowmik. 2006. The biology of invasive alien plants in Canada. 5. Polygonum cuspidatum Sieb. & Zucc. (Fallopia japonica [Houtt.] Ronse Decr.). Canadian Journal of Plant Science, 86(3):887-905.
Braatne, J.H., S.M.P. Sullivan, and E. Chamberlain. 2007. Leaf decomposition and stream macroinvertebrate colonisation of Japanese knotweed, an invasive plant species. International Review of Hydrobiology, 92(6):656-665.
Bram, M.R. and J.N. McNair. 2004. Seed germinability and its seasonal onset of Japanese knotweed (Polygonum cuspidatum). Weed Science, 52(5):759-767.
Dassonville N., S. Vanderhoeven, W. Gruber, and P. Meerts. 2007. Invasion by Fallopia japonica increases topsoil mineral nutrient concentrations. Ecoscience, 14(2):230-240.
Dorn, R. 1984. Vascular plants of Montana. Mountain West Publishing, Cheyenne, Wyoming.
Francis, R.A., K.A. Riley, and S.P.G. Hoggart. 2008. Vegetative regeneration of Fallopia japonica (Houtt.) Ronse Decraene (Japanese knotweed) at varying burial depths. Weed Biology and Management, 8(1):69-72.
Gaskin, J.F., M. Schwarzlander, F.S. Grevstad, M.A. Haverhals, R.S. Bourchier, and T.W. Miller. 2014. Extreme differences in population structure and genetic diversity for three invasive congeners: knotweeds in western North America. Biological Invasions, 15:2127-2136.
Hirose, T., and M. Tateno. 1984. Soil nitrogen patterns induced by colonization of Polygonum cuspidatum on Mt. Fuji. Oecologia, 61:218-223.
Hitchcock, L., A. Cronquist. 1973. Flora of the Pacific Northwest. University of Washington Press, Seattle.
Inoue, M., H. Nishimura, H.H. Li and J. Mizutani. 1992. Allelochemicals from Polygonum sachalinense (Polygonaceae). Journal of Chemical Ecology, 18(10):1833-1840.
Kala, C.P. 2004. Pastoralism, plant conservation, and conflicts on proliferation of Himalayan knotweed in high altitude protected areas of the Western Himalaya, India. Biodiversity and Conservation, 13(5):985-995.
Kurose, D., N. Furuya, M. Matsumoto, D.H. Djeddour, H.C. Evans, and K. Tsuchiya. 2009. Evaluation of a Puccinia Rust as a Potential Biological control agent of Fallopia japonica. Journal of the Faculty of Agriculture Kyushu University, 54(1):59-64.
Lacerf, L., D. Patfield, A. Boiche, M. Riipinen, E. Chauvet, and M. Dobson. 2007. Stream ecosystems respond to riparian invasion by Japanese knotweed (Fallopia japonica). Canadian Journal of Fisheries and Aquatic Sciences, 64(9):1273-1283.
Locandro, R.R. 1978. Weed Watch: Japanese Bamboo. Weeds Today. Fall. pp. 21-22.
Lynn, L.B., R.A. Rogers, and J.C. Graham. 1979. Response of woody species to glyphosate in northeastern United States. Proceedings Northeast Weed Science Society. 33:336-342.
Maerz, J.C., B. Blossey, and V. Nuzzo. 2005. Green frogs show reduced foraging success in habitats invaded by Japanese knotweed. Biodiversity and Conservation, 14(12):2901-2911.
Mandak, B. P. Pysek, and K. Bimova. 2004. History of the invasion and distribution of Reynoutria taxa in the Czech Republic: a hybrid spreading faster than its parents. Preslia, 76(1):15-64.
Scott, R., and R.H. Mars. 1984. Impact of Japanese knotweed and methods of control. Aspects of applied biology 5, Weed control and vegetation management in forestry and amenity areas. pp. 291-296.
Seiger, L.A. 1991. Elemental stewardship abstract for Polygonum cuspidatum Japanese knotweed, Mexican bamboo. The Nature Conservancy. Arlington, Virginia.
Seiger, L.A. and H.C. Merchant. 1997. Mechanical control of Japanese knotweed (Fallopia japonica [Houtt.] Ronse Decraene): Effects of cutting regime on rhizomatous reserves. Natural Areas Journal, 17(4):341-345.
United States Department of Agriculture Natural Resource Conservation Service Plants Database. http://plants.usda.gov/. Accessed November 2010.
Urgenson, L.S., S.H. Reichard, and C.B. Halpern. 2009. Community and ecosystem consequences of giant knotweed (Polygonum sachalinense) invasion into riparian forests of western Washington, USA. Biological Conservation, 142(7):1536-1541.
Wang, Y., J.Q. Ding, and G. Zhang. 2008. Gallerucida bifasciata (Coleoptera: Chrysomelidae), a potential biological control agent for Japanese knotweed (Fallopia japonica). Biocontrol Science and Technology, 18(1):59-74.
Wilson, L.M. 2007. Key to identification of invasive knotweeds in British Columbia. B.C. Ministry of Forest and Range, Forest Practices Branch, Kamloops, B.C. http://www.for.gov.bc.ca/hfp/biocontrol/downloads/Knotweed_key_BC_2007.pdf
ACKNOWLEDGEMENTS
This bulletin was produced with suggestions from Jerry Marks, Tim Miller, Linda Wilson, and Noelle Orloff. Special thanks to Susan Anderegg for assistance with layout and design. Funding assistance was provided by the Montana Noxious Weed Trust Fund. Reprinting in 2017 was made possible with a grant from USDA-National Institute of Food and Agriculture.
TABLE 1. Characteristics of species in the knotweed complex. (Adapted from Key to Identification of Invasive Knotweeds in British Columbia. Wilson 2007.)
| Giant Knotweed | Bohemian knotweed | Japanese knotweed | Himalayan knotweed | |
| Plant Size | 9'9" to 19'8"(3-6m) | 6'6" to 16'5"(2-5m) | 4'10" to 8'2"(1.5-2.5m) | 6'6" to 9'10"(2-3m) |
Leaf Size |
7.8 to 16" long, (20- 40cm) 2/3 as wide | 2 to 12" long,(5-30cm) 2/3 as wide | 1 to 4" long, (3-10cm) 2/3 as wide | up to 8" long, (20cm), less than 1/2 as wide |
Sex |
Perfect and fertile, usually produces seed | Female or perfect, occasionally produces seed | Female or perfect (rare), occasionally produces seed | Perfect and fertile, usually produces seed |
Flower color & arrangement |
Greenish-white to creamy-white in a compact, drooping arrangement | Greenish-white to creamy-white in an erect or loose, drooping arrangement | Greenish-white to creamy- white in a loose, drooping arrangement | Pinkish-white to pink in aloose, spreading arrangement |
If you believe you’ve found a plant in the knotweed complex, please contact your local Extension or weed district office, or send a sample to: Montana State University, Schutter Diagnostic Laboratory, 121 Plant BioScience Building, Bozeman, MT 59717-3150.



