Nitrogen Cycling, Testing and Fertilizer Recommendations
This is the third in a series of Extension materials designed to provide pertinent information on a variety of nutrient management, water management, and water quality issues to Extension agents, Certified Crop Advisers (CCAs), consultants, and producers. We have included a series of questions at the back of this module that will make the learning “active” as well as offer the potential for CEU credits for CCAs. In addition, we have included a resource section of other Extension materials, books, web sites, and professionals in the field.
Last Updated: 06/05by Clain Jones and Jeff Jacobsen
Objectives
- Describe the 9 major nitrogen processes that occur in soil
- Describe the major factors that affect each of the nitrogen processes
- Recognize how different crops and cropping systems affect N availability
- Understand optimum nitrate sampling depths for different conditions
- Understand how a soil nitrate test result is used to estimate N fertilizer requirements
- Calculate N fertilizer application rates
Background
Nutrient Management Module 2 discussed the 14 mineral nutrients that are required by plants for growth and reproduction. Of these, nitrogen (N) is generally taken up in larger amounts than the others and is the most common, and most important, limiting nutrient for agricultural crops. Not only does N affect yield, but it also affects the quality (protein or sugar content) of crops such as wheat, barley, and sugar beets. In addition, N also interacts with most of the other macronutrients. To understand how cropping systems, N fertilizer forms, application rates, and timing of N fertilization affect crop yield and quality, it is important to first understand the various transformations that N undergoes within the soil.
Nitrogen Cycling
Of all the mineral nutrients, N has the most complex nutrient cycle, largely because N can exist as a gas (both ammonia and nitrogen gas), whereas the other 13 mineral nutrients do not exist as gases under normal soil conditions. To help understand the various components of the N cycle, definitions and molecular formulas of the numerous N forms are provided in Table 1. Available N is generally considered to be the sum of ammonium and nitrate, although urea, a type of organic N, may also be plant available.
Nitrogen cycling consists of nine major processes: plant uptake, exchange, nitrification, denitrification, volatilization, mineralization, immobilization, N2 fixation, and leaching (Figure 1). Each of these processes, and the effect that each has on plant available N (and hence yield), is described below. As you read about each of these processes, think about how each will affect the amount of nitrate and ammonium in different soil systems because these two forms are available to plants, and therefore, can directly affect crop yield.
Table 1. Definitions of each N form.
| Nitrogen Form | Molecular Formula | Notes |
| Nitrogen gas | N2 (g) | Represents about 80% of the air we breathe |
| Ammonia gas | NH3 (g) | Generally cheapest form of N, toxic at high concentrations |
| Ammonium | NH4+ | Plant available, attracted to exchange sites on clay particles |
| Nitrate | NO3- | Very mobile, requires more energy by plant than ammonium |
| Nitrite | NO2- | Mobile, generally low concentrations, toxic to young mammals |
| Organic N | - | Slowly supplies available N to soil solution |
Plant Uptake
Annual crop uptake of N can vary from approximately 50 to 200 lb/ac per year, depending on crop and yield (Table 2). N uptake can be estimated by dividing a grower’s yield by the yield shown in the table, and then multiplying this amount by the N uptake. A more accurate approach is to multiply plant tissue N content (as a fraction) by dry yield (in lb/ac). It’s useful to compare actual uptake rates to N fertilizer rates, because N fertilizer rates that are much higher (two-fold or more) than N uptake suggest an excessive loss of N and a possible need to refine N application rates or management practices. The amount of N uptake will be largely controlled by the concentration of available N in the soil, a quantity that is controlled by the processes described below.
Exchange
Cation exchange was described in Nutrient Management Module 2. Briefly, ‘exchange’ indicates that ions (charged molecules) are attracted to a soil surface. Because clays generally have negative charges, and ammonium (NH4+) has a positive charge, it will be attracted to, and held weakly on clay particles. The general term for this process is ‘sorption,’ which prevents ammonium from moving very rapidly through the soil. Although it may seem that the ammonium would not be available for plant uptake, ammonium can move away from the soil surface as ammonium levels decrease in soil solution due to the process known as diffusion (see NM Module 2). Hence, there is an exchange of ammonium between soil and soil solution. More ammonium is held by high pH (neutral to alkaline) soils, and conversely, ammonium moves more readily in low pH (acidic) soils.
The two negatively charged N forms (nitrate and nitrite) will be repelled from negative charges on the clay surface, and are not attracted very strongly to the fewer positive charges on clay surfaces. Therefore, nitrate and nitrite both have relatively high mobility, meaning they can move easily through the soil and do not undergo much exchange. In addition, nitrate requires more energy by the crop after it is taken up because the nitrate must be converted to ammonium in the plant before it is made into proteins. Unfortunately, in well-aerated agricultural soils, ammonium readily converts to nitrate in a process called nitrification, which is described on the next page.
Figure 1. The Nitrogen Cycle.
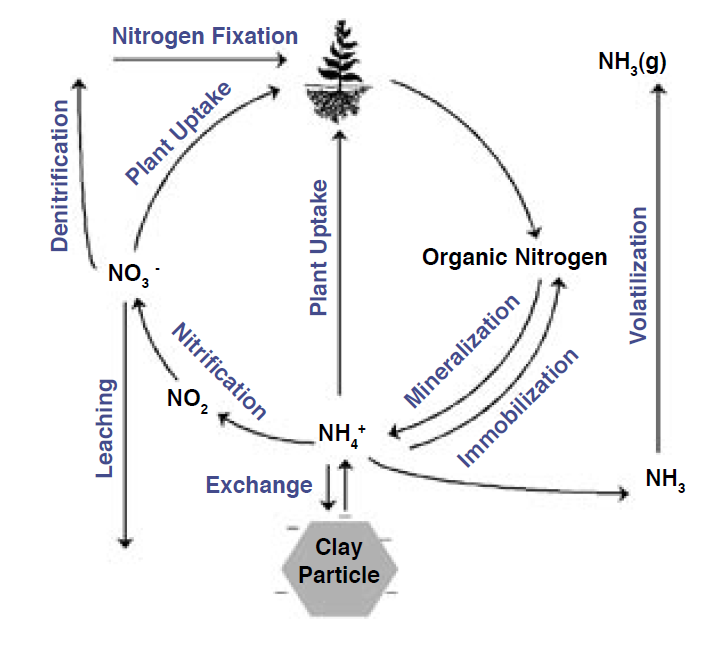
Table 2. N uptake amounts in harvested portions of selected agricultural crops.
| Crop | Assumed Yield Per Acre | N Uptake (lb/ac) |
| Alfalfa | 2.5 t | 150 |
| Barley | 50 bu | 80 |
| Brome | 1.5 t | 66 |
| Corn silage | 20 t | 167 |
| Oats | 60 bu | 70 |
| Orchard grass | 1.5 t | 75 |
| Potatoes | 300 cwt. | 162 |
| Sugar Beets | 25 t | 210 |
| Timothy | 1.5 t | 56 |
| Wheat | 40 bu | 70 |
Adapted from CFA (1995).
Q&A #1
It sounds like it would be beneficial to stop or slow nitrification to prevent leaching losses. Are there any products that do this?
There are two labeled compounds (nitrapyrin and dicyandiamide) designed to inhibit nitrification as of the year 2000 in the U.S. (Hoeft et al., 2000).
However, they are not widely used in Montana or Wyoming, and research on the effectiveness of nitrification inhibitors is mixed (Prasad and Power, 1997).
Nitrification
Soil ammonium (NH4+) can quickly (hours to weeks) be converted into nitrite (NO2-) and then into nitrate (NO3-). This process, known as nitrification, only occurs in the presence of oxygen, so generally it will be slow or non-existent in water logged, anaerobic soils. Notice that the N in NH4+ loses H+, lowering pH, and gains oxygen (O) during nitrification. The microorganisms that convert ammonium to nitrite and then to nitrate are called nitrifiers or nitrifying bacteria. The second part of this process, the conversion of nitrite to nitrate, occurs very rapidly in all but saturated soils. This is fortunate because nitrite is toxic to both plants and animals.
Nitrification occurs most rapidly at pH levels between 6.5 and 8.5, at soil temperatures between about 75 and 95oF, and at moisture contents above the wilting point. Nitrification occurs rapidly in most agricultural soils, because they are generally well aerated, near neutral pH, and have warm temperatures. Interestingly, anhydrous ammonia fertilizer undergoes nitrification somewhat slower than other ammonia fertilizer materials because the high pH and ammonia in the band (explained later) inhibit the nitrifying bacteria. As pointed out above, it would be desirable if nitrification occurred more slowly, because nitrate can be leached out of the soil profile, whereas ammonium generally stays in the soil profile and is readily available for plant uptake and utilization.
Denitrification
Denitrification refers to the process where nitrate (NO3-) becomes nitrogen gas (N2(g)). It is the opposite of nitrification in that oxygen is removed rather than added. Denitrification requires the absence of oxygen, or ‘anaerobic’ conditions. Poorly drained soils can result in a 4-5% nitrate loss per day, possibly causing substantial yield losses (Hoeft et al., 2000). Similar to nitrification, microorganisms are responsible for denitrification, and therefore it occurs faster in warm, moist soils. Recall from the discussion on nitrification that nitrate can only form in the presence of oxygen, whereas denitrification requires that nitrate be present and there be no oxygen. Therefore, denitrification losses of N are most significant when soils alternate between aerobic conditions, which allow nitrate to accumulate, and anaerobic conditions. In fine textured soils, this could occur in a flood-irrigated field. It can also occur in fields with shallow groundwater tables, especially during irrigation cycles or oscillating dry and wet periods.
Interestingly, denitrification has been found to occur in soils containing 5% oxygen (air contains about 20% oxygen). How is that possible if denitrifying organisms require anaerobic conditions? The answer is that there are small pores within the soil that can be saturated and anaerobic. These anaerobic ‘microsites’ have been found to result in substantial levels of denitrification even in surface soils (Havlin et al., 1999), although the amount of denitrification that occurs in Montana and Wyoming soils is not known.
Denitrification is increased in soils that have readily decomposable organic matter because denitrifying organisms rely on organic matter for energy. Plants have been found to increase denitrification rates likely because of the release of readily available organic matter from roots and root tissue. Denitrification increases with temperature between 40 and 80oF, and is relatively constant above 80oF (Havlin et al., 1999). It is inhibited at pH levels below 5.6, but is relatively constant from pH 5.6 to 8, which encompasses the vast majority of Montana and Wyoming soil pH levels. Using practices that prevent waterlogged soils is probably the best way to ensure that denitrification losses are minimal.
Volatilization
Ammonia (NH3) volatilization refers to the loss of ammonia as a gas into the atmosphere, and can be a source of N loss. The process is increased at high pH because NH4+ will more easily convert to NH3 at high pH. Therefore, those fertilizers that increase pH further (urea and anhydrous ammonia) may increase ammonia volatilization. This is less of an issue in well-buffered soils, because the fertilizer cannot increase the pH as substantially as in poorly buffered soils. Buffering refers to the soil’s ability to resist change; for example, clay soils are generally better buffered than sandy soils, and calcareous soils are generally highly buffered. Because ammonia needs to be in contact with air to volatilize, incorporating ammonia-based fertilizers into the soil will also substantially decrease volatilization potential and increase yields. Volatilization increases with increasing wind, increasing temperature (up to about 110oF), soil coarseness (likely due to better gas flow), and N fertilizer application rates. Cool temperatures and generally well-buffered soils in Montana and Wyoming may be two reasons that researchers and producers in this region have not noticed substantial losses of surface applied urea (Jackson, Jacobsen unpub. data).
Applying anhydrous ammonia in very dry or very wet soils can increase volatilization, because the soil will not quickly seal behind the injector knife, allowing the vapor to escape. Volatilization has been observed to occur the slowest between 15 and 20% moisture in a loam soil (Prasad and Power, 1997). Applying ammonia-based fertilizer immediately before a rainstorm can help push it further into the soil profile where it is less available for volatilization. The best methods to decrease volatilization are to incorporate fertilizers, apply during calm and cool periods, and if possible, use split applications to decrease application rates (Table 3). Keep in mind from the above that ammonium converts to nitrate (nitrification) in hours to weeks, and once N becomes nitrate, it can no longer volatilize.
Table 3. Optimum conditions to minimize ammonia volatilization losses.
| Factor | Optimum |
| Temperature | Low |
| Wind | Calm |
| Moisture | 15-20% |
| Soil texture | Fine |
| Fertilizer placement | Incorporated |
| N application rates | Low |
Mineralization
As microorganisms decompose organic matter, ammonium is released in a process called mineralization. The amount of N converted from organic forms to available forms by mineralization ranges from approximately 13 to 62 lb/acre per year (Pierzynski et al., 2000). Mineralization amounts are higher in soils with higher amounts of organic matter; therefore, taking steps to maintain or increase soil OM (with no-till, minimum till, or organic additions) can help supply a relatively constant amount of available N to the soil. As a rule of thumb, 20-30 lb N/ac is mineralized per 1% OM. However, because mineralization requires microorganisms, it is highly affected by soil conditions. For example, mineralization occurs optimally in aerobic, moist, and warm soil, with near neutral pH levels.
The amount of mineralization is also dependent on the type of organic matter present. Fresh manure or crop residue will break down faster than humus that is the result of years of decomposition. In addition, the ratio between total soil carbon (C) and total soil N affects how quickly this process occurs, because micro-organisms, like plants, need N to live. For example, when the C:N ratio (i.e., total C divided by total N) is less than about 30:1, net mineralization generally occurs (Pierzynski et al., 2000). At C:N ratios less than 20:1, ammonia tends to accumulate, which can volatilize and cause odor. For those who apply organic materials, such as manure, sludge, or straw, it is especially important to try to optimize mineralization to avoid depleting available N in the soil (if too high a C:N is used) and to possibly avoid excessive odor, if this is a potential concern. C:N ratios of various organic materials are shown in Table 4. Note that the materials with low C:N ratios are generally more odorous, yet will break down more quickly than those materials with high C: N ratios. Different organic amendments can be mixed to obtain a desired C:N ratio.
Q&A #2
It looks like crop residues have C:N ratios much higher than soil organic matter. What makes the C:N of crop residues go down as they decay?
Microorganisms convert organic carbon to CO2 gas, which goes into the air, but N stays in the soil. Therefore, C levels decrease, and N levels stay about the same, causing the ratio of C:N to decrease.
Table 4. Carbon to nitrogen (C:N) ratios of various organic materials.
| Organic Material | C:N |
| Raw municipal wastewater | 5 |
| Treated municipal sludge | 10 |
| Soil organic matter | 10 |
| Sweet clover | 12 |
| Poultry manure | 16 |
| Steer manure | 20 |
| Rye | 36 |
| Corn roots | 48 |
| Corn/sorghum stover | 60 |
| Straw | 80 |
| Sawdust | 400 |
Immobilization
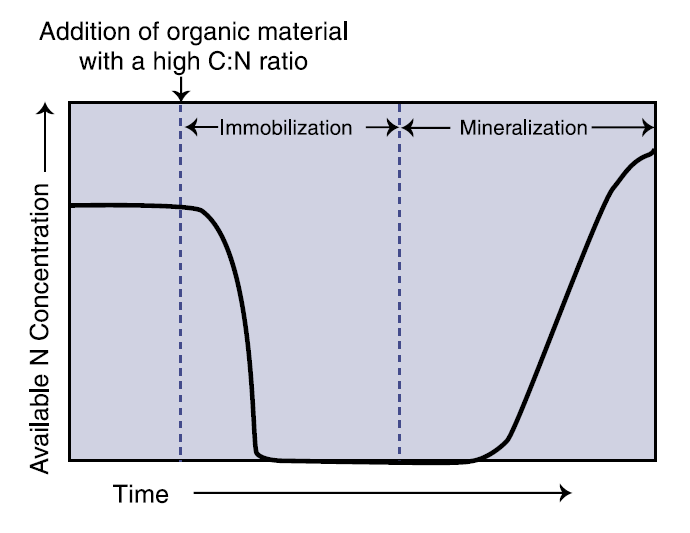
N immobilization refers to the process where inorganic N (NO3- or NH4+) is biologically converted to organic N, and is essentially the reverse of mineralization. Microorganisms immobilize N by taking it up and converting it into proteins and cell walls. By definition, plants also immobilize N, but immobilization more commonly refers to the process where micro-organisms remove available N from solution. As you may expect, immobilization occurs more easily at high C:N ratios (above 30:1) because microorganisms scavenge any available N in the soil as they help break down the relatively N-free organic material (Figure 2). Plant growth can be substantially stunted following the addition of a high C:N material unless N fertilizer is added to offset the depletion of available N. It can take from four to eight weeks for available N levels to begin to climb after addition of a high C:N crop residue or amendment (Havlin et al., 1999), although the time is dependent on all the factors listed previously that affect microbial health such as soil water, available N, and temperature. Note that available N concentrations can eventually be greater than initial N concentrations, even with the addition of a high C:N organic material such as tilled-in grain stubble.
Because immobilization is controlled by microorganism growth, it occurs most readily in warm, moist soils with near neutral pH levels. The best way to avoid substantial amounts of N immobilization is to avoid large applications of high C:N organic materials, or to supplement such additions with N fertilizers. Also recall from Figure 2 that immobilized N will eventually become mineralized as microorganisms die and decompose, increasing available N levels. Therefore, immobilization is not necessarily a negative outcome, especially if it can be timed with a period when a field is fallow, thereby capturing the N in a form that is not leachable.
Nitrogen Fixation
Nitrogen gas (N2(g)) can be converted into available forms of N through the process known as ‘nitrogen fixation.’ There are three major N fixation processes: ammonia fertilizer production, lightning, and biological fixation. Ammonia fertilizers require natural gas, steam, oxygen, and a catalyst to fix N2(g). Therefore, ammonia fertilizer prices are heavily dependent on the price of natural gas. Lightning also fixes N, although the amount of available N that reaches the earth from the atmosphere is generally less than 5 lb/acre per year (Brady, 1984).
Figure 2. Available N changes following addition of high C:N organic material.
Some organisms are able to convert atmospheric N2(g), which represents approximately 80% of the air we breathe, into ammonium. Worldwide, biological N2 fixation is estimated at 145 to 200 million tons per year, compared to approximately 90 million tons per year of world fertilizer use (Havlin et al., 1999). In crop production in the U.S., the amount of biological N2 fixation is approximately 1/3 of the amount of fertilizer N applied (Havlin et al., 1999). ‘Symbiotic’ N2 fixation occurs when a bacterium, such as Rhizobium, ‘infects’ a root hair of a legume, such as alfalfa. The root hair wraps around the bacterium, creating a nodule on the root (Figure 3). The bacteria trapped inside the nodule continue to multiply and fix N2 that is in the soil. Nodules are generally pink to salmon color when the organisms are actively fixing N2(g) due to a compound called ‘leghemoglobin,’ which contains iron, and is similar to hemoglobin in human blood.
Figure 3. Bacteria nodules on bean roots.
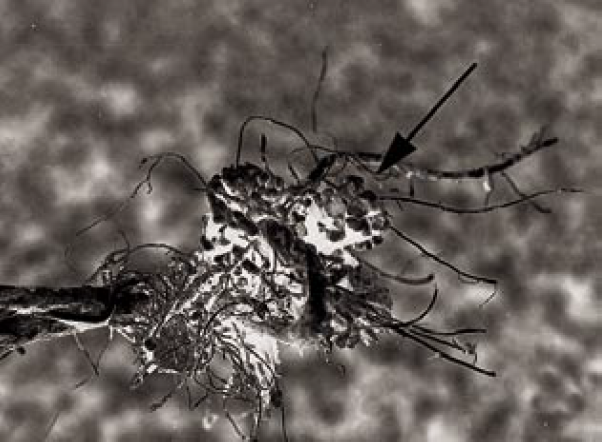
Figure 4. Effect of inoculation on nodulation and bean health. Plant on left was not inoculated, causing N deficiency.

Q&A #3
What do bacteria get from the plant in exchange for providing N to the plant?
The bacteria receive carbon from the plant, which it needs for energy and growth. The loss of carbon from the plant can be considerable and is why the plant does not assist with fixation in a high available N environment. In addition, the nodule provides a controlled, low oxygen, environment that allows the N2 fixation to occur.
Symbiotic N2 fixation is affected by many factors, including nutrient content, inoculation, soil pH, moisture, and plant health. Symbiotic N2 fixation is slowed by a lack of calcium, phosphorus, cobalt, boron, iron, copper, or molybdenum. In addition, high levels of available N can greatly diminish N fixation because the plant stops releasing a chemical that attracts the bacteria to the roots, and the plant does not allow nodules to form. Specifically, in a mixed grass-legume stand, N fertilization with up to 100 lb N/ac significantly decreased legume yield, significantly increased grass yield, and had no effect on total yield above 33 lb N/ac (Tueller, 1988). Essentially, high levels of N fertilization favor grass over legumes, decreasing the amount of N that the legumes supply to the stand, and converting the stand to primarily grass. Therefore, fertilizing pure legumes or legume-grass stands with more than 30 to 40 lb N/ac is generally not recommended, although in some grass-alfalfa stands, additional N can be a benefit for the second and third cuttings. Keep in mind that phosphorus fertilizer requirements are generally met by the addition of ammonia phosphate fertilizer materials, so some N is often supplied with P. For more information on the effects of excess nitrate on legumes, see Nitrate Poisoning of Legumes (MT9801AG-see Appendix for ordering information).
Each leguminous plant (called the ‘host’ plant) has a different strain of bacteria that fixes N2. Therefore, that particular type of bacteria either needs to be in the soil, or added with the seed, a step called ‘inoculation.’ For example, the bacteria species that inoculates alfalfa will not work with beans, and vice versa. The positive effects of inoculating legumes on plant health can be dramatic (Figure 4).
N2 fixation inhibited by pH levels below 6 for alfalfa and 5 for red clover. Legume roots and N2 fixing bacteria can both be injured by high levels of aluminum and manganese, which are elevated at low pH levels. Therefore, liming low pH soils can help increase N2 fixation in legumes. N2 fixation is also increased when photosynthetic activity is increased, likely because the N2 fixing organisms obtain more carbon when photosynthesis levels are high (Q&A #3). Therefore, adequate moisture and warm temperatures will generally increase N2 fixation.
Not only does N2 fixation supply N to the microorganism and plant, but it can also increase available N levels in the soil for years following a legume crop. This is why rotating legumes with grains can be an attractive cropping strategy. For example, in a study of dryland wheat-legume rotations, wheat yield was 38 bu/ac for a wheat-field pea rotation compared to 32 bu/ac for continuous wheat (Miller et al., 1998). In addition, wheat grown following peas had a protein level of 13% compared to 12.1% for continuous wheat. Barley also shows increased yield following a pea crop, with especially significant yield increases at low fertilizer N rates (Figure 5). The difference in yields between barley grown in wheat versus canola stubble is attributed to differences in pest pressure.
In addition to the symbiotic N2 fixation discussed above, there are also bacteria that fix N2 that are not attached to roots. Generally, these ‘free-living’ bacteria are not believed to add more than about 5 lb N/ac to most agricultural soils (Havlin et al., 1999).
Leaching and Upward Movement
An available N ‘mass balance,’ or summary of inputs and outputs, should be starting to form, meaning we’ve looked at N gains (release from exchange sites, mineralization, and biological N2 fixation) and N losses (plant uptake, sorption to exchange sites, denitrification, volatilization, and immobilization) to the available N pool. In addition, N fertilizer, irrigation, manure, and precipitation (<5 lb/ac-year), represent other inputs to the pool (Table 5). The final potential loss from the soil system is nitrate leaching.
Figure 5. Yield of barley grown in pea, canola, and wheat stubble. Modified from Beckie and Brandt (1997).
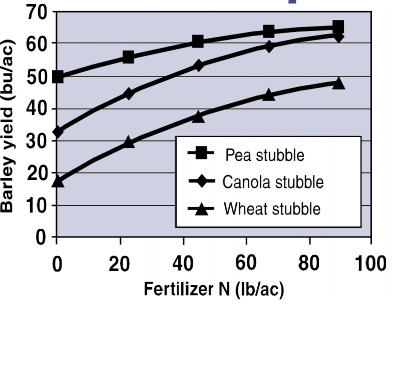
Nitrate is highly mobile as discussed previously and in Nutrient Management Module 2. The areas with the highest risk for nitrate leaching are associated with high precipitation, irrigated conditions, or coarse textured, shallow soils. Nitrate leaching, like denitrification and volatilization, represents an economic loss, because once the N has left the soil system, it is not available for crop uptake. In addition, because of health concerns with nitrate in groundwater, nitrate is regulated by the U.S. Environmental Protection Agency (USEPA). In Montana, there are several counties where average nitrate levels in groundwater approach the USEPA drinking water standard of 10 ppm (mg/L) as N in drinking water, and the majority of counties located east of the continental divide had at least one well exceeding this standard (Bauder et al., 1993). Factors that were correlated with high groundwater nitrate concentrations in Montana included coarse soil textures, low slopes, dryland crop production, tilled cropland, and summer fallow. The lack of N and water uptake during summer fallow periods likely increases nitrate leaching. Practices that increase crop uptake, and decrease excessive percolation, should minimize the amount of nitrate leaching.
Table 5. Available N gains and losses in the soil.
| Gains | Losses |
| Release from exchange sites | Sorption to exchange sites |
| Mineralization | Immobilization |
| Biological fixation | Denitrification |
| Fertilizer | Volatilization |
| Precipitation | Plant uptake |
| Irrigation water | Leaching |
| Manure |
Nitrate can also move upward, especially in semi-arid and arid regions. Upward movement of nitrate and other soluble ions occurs when evaporation exceeds precipitation, causing water to move upwards. The ease that nitrate moves either upward or downward affects soil sampling methods for nitrate as described below.
Q&A #4
Why is nitrate in groundwater a concern?
Nitrate can cause a disease referred to as methemoglobinemia, or blue-baby disease. Infants, as well as young livestock, have a different type of hemoglobin than adults. If infants ingest water, food, or milk with excess nitrate and nitrite, oxygen is pulled from their bloodstream, depriving them of necessary oxygen.
Soil Sampling for Nitrate
Soil sampling methods and laboratory selection were described in detail in Nutrient Management Module 1. This section briefly describes special considerations for the sampling and testing of soil N. Generally, only soil nitrate, and not ammonium, is sampled in soils because ammonium is converted so quickly to nitrate via nitrification in agricultural soils that ammonium levels are generally much lower than nitrate levels. Because nitrate is very mobile in soils, and can move upward as pointed out above, sampling just the upper 6 inches is generally not a good indicator of the total amount of nitrate available to the plant-root system. Nitrate N should be sampled to 3 feet where possible, and up to 4 feet for deep rooted crops such as sugar beets and wheat, if a truck-mounted probe is available. Generally, the top 6 inch sample and the 6- to 24-inch sample will be analyzed for nitrate N. Samples greater than 24 inches can be composited and also submitted.
The laboratory will generally calculate the total nitrate in lb/ac, although if the data is reported in ppm, the conversion to lb/ac can be performed as shown (Calculation Box 1). The factor of 2 in the equation is derived from the assumption that an acre-furrow slice (6 inch slice) of soil weighs 2 million pounds. This number is somewhat higher in soils with above average ‘bulk densities,’ which is the case with compacted soils, and is somewhat lower with soils high in organic matter or that have been recently plowed. The bulk density is simply the dry weight of the soil divided by the volume, and is usually expressed in pounds per cubic foot (lb/ft3).
Calculation Box 1
CALCULATION: NITRATE-N (lb/acre) = NITRATE-N CONCENTRATION (ppm) X 2 X SAMPLE THICKNESS/6”
Example: 0-6 inch 8 ppm NO3-N (or nitrate-N, meaning nitrate expressed as N in ppm)6-24 inch 4 ppm NO3-NN in 0-6 inch increment = 8 x 2 x 6”/6” = 16 lb/acreN in 6-24 inch increment = 4 x 2 x 18”/6” = 24 lb/acreN total in 0-24 inch profile = 40 lb/acre
Sampling deeper than 24 inches is not generally possible with a hand probe, but if a truck-mounted probe is available, deeper samples can provide useful information. For example, if a bulk of the soil nitrate is below 2 feet, and it’s believed that much of this will be available to the crop, the fertilizer N recommendation can be decreased. Soils can be broken into even more sections, especially when sampled deeper than 2 feet. This provides the professional making fertilizer recommendations with more information that will help to fine-tune the recommendation. For example, if the bulk of the soil nitrate was near the bottom of the soil profile, the soil was coarse and moist, and heavy precipitation had fallen since the time of sampling, it’s possible that much of the deep nitrate leached out of the profile and should not be included in the calculations of soil profile nitrate.
Table 6. Spring wheat N fertilizer guidelines for Montana.
|
Yield Potential
bu/acre
|
Soil NO3-N + Fertilizer N
lb N/acre |
| 30 | 99 |
| 40 | 132 |
| 50 | 165 |
| 60 | 198 |
| 70 | 231 |
| 80 | 264 |
Conversely, in a dry year, some nitrate may not become available if roots cannot penetrate some dry sections of the soil. In either case, N fertilizer recommendations may be increased somewhat. Growers should sample following periods of drought to assess the soil nitrate levels since these levels tend to accumulate during periods of below average yields. The following section introduces the science, and art, of making accurate N fertilizer recommendations.
Calculation Box 2
CALCULATE THE N FERTILIZER REQUIREMENT FOR SPRING WHEAT THAT HAS A YIELD POTENTIAL OF 50 bu/ac
ASSUME SOIL N = 40 lb/ac AS SHOWN IN CALCULATION #1
Recommended Soil NO3-N + Fertilizer N = 165 lb/ac (from Table 6)Fertilizer N = 165 lb/ac – Soil NO3-NFertilizer N = 165 lb/ac – 40 lb/acFertilizer N = 125 lb/acFertilizer needed = Fertilizer N/fraction of N in fertilizerUrea needed = (125 lb/ac)/0.46 = 272 lb/ac
N Fertilizer Recommendations
There are a number of strategies for determining N recommendations including historical amounts, budget inventories of gains and losses, and using yield-response curves. The strategies used by the different laboratories that serve Montana and Wyoming are listed in MSU Extension Bulletin 150 (ordering information is in the appendix). Keep in mind that fertilizer recommendations supplied by laboratories can vary based on their philosophies and databases, and should therefore be reviewed carefully (see NM Module 1). Budget inventories generally assume an N mineralization amount (based on organic matter content of the soil), previous crop contributions, residual (nitrate) N, and yield goal. Fertilizer Guidelines for Montana Crops (EB 161) are based on applied research in the Northern Great Plains, and require yield potential and soil NO3-N to 2 feet (Table 6). The guidelines are currently being revised based on ongoing research, and Table 6 reflects revised spring wheat guidelines. The yield potential is generally based on past yields and can be adjusted based on soil moisture. Sometimes the yield potential is assumed to equal an amount 5 to 10% higher than average historical yields. The higher yields maybe realistic if, for example, plans call forseeding with higher yielding cultivars. The soil NO3-N, or ‘residual nitrate’ is either provided by the laboratory or calculated as was shown in Calculation Box 1. An example fertilizer N calculation is shown in Calculation Box 2 (previous page). Note that the fertilizer guidelines recommend approximately 3.3 lb N/bu of yield potential for spring wheat. This value is sometimes used instead of the tables. Keep in mind that fertilizer guidelines are 1) guidelines that should be adjusted based on your region and historical results and 2) often designed to optimize yield, not quality. Recent research has shown that 3.2 lb N/bu is needed at yield potentials between 40 and 60 bu/ac to produce winter wheat with 14% protein, a protein level that pays a premium (Jackson, 2001).
Once a fertilizer N requirement is determined, the amount of fertilizer to apply can be calculated by knowing the fraction, or percentage, of N in the fertilizer to be used (Table 7). For example, urea (CO(NH2)2) has an analysis of 46-0-0, meaning it contains 46% N, 0% P2O5, and 0% K2O. Therefore, the fraction of N in urea is 0.46 (46/100), and the amount of urea needed can be calculated as shown in Calculation Box 2. Additional information on the pros and cons of various N fertilizers, application methods, and timing of fertilizer application will be covered in a future module.
Summary
N can undergo numerous transformations in the soil that either make it more, or less, available to plants. Some of these processes cannot be altered by producers, but are instead controlled by soil factors such as soil texture and temperature. Some of these processes, however, can be affected by different management practices, such as tillage, irrigation, and residue management. By understanding the various factors that affect the N cycle, N losses can be minimized and yields optimized.
Soil samples for N should be collected as deep as possible due to nitrate’s high mobility, and hence availability, in soils. N fertilization recommendations are generally supplied by laboratories, but should be verified by using published fertilizer guidelines and publications.
References
Bauder, J.W., K.N. Sinclair, and R.E. Lund. 1993. Physiographic and Land Use Characteristics associated with nitrate nitrogen in Montana groundwater. J. Environ. Qual. 22:255- 262.
Beckie, H.J., and S.A. Brandt. 1997. Nitrogen contribution of field pea in annual cropping systems. 1. Nitrogen residual effect. Can. J. Plant Sci. 77:311-322.
Brady, N.C. 1984. The Nature and Properties of Soils. 9th Edition. Macmillan Publishing Company New York. 750 p.
CFA. 1995. Western Fertilizer Handbook. 8th ed. California Fertilizer Association. Interstate Publishers, Inc. Danville, Illinois. 338 p.
Havlin, J.L., J.D. Beaton, S.L. Tisdale, and W.L. Nelson. 1999. Soil Fertility and Fertilizers. 6th Edition. Prentice Hall. Upper Saddle River, NJ. 499 p.
Hoeft R.G., E.D. Nafziger, R.R. Johnson, and S.R. Aldrich. 2000. Modern Corn and Soybean Production. MCSP Publications. Champaign, IL.
Jackson, G.D. 2001. Fertilizing winter wheat with nitrogen for yield and protein. Fertilizer Fact Sheet 26. MSU Extension Service and Agricultural Experiment Station, Bozeman, MT.
Miller, P., R. Zentner, B. McConkey, C. Campbell, D. Derksen, C. McDonald, and J. Waddington. 1998. Using pulse crops to boost wheat protein in the Brown soil zone. p. 313-316. In D.B. Fowler et al. (ed.) Wheat Protein Production and Marketing. Proc. Wheat Protein Symposium, Saskatoon, Saskatchewan. 9-10 March, 1998. University Extension Press, Saskatoon, Saskatchewan, Canada.
Pierzynski, G.M., J.T. Sims, and G.F. Vance. 2000. Soils and Environmental Quality. 2nd Ed. CRC Press. Boca Raton, FL. 459 p.
Prasad, R. and J.E. Power. 1997. Soil Fertility Management for Sustainable Agriculture. CRC Press, Boca Raton, FL. 356 p.
Tueller, P.T. 1988. Vegetation Science Applications for Rangeland Analysis and Management. Kluwer Academic Publishers. Norwell, MA.
Appendix
Books
Western Fertilizer Handbook. 9th Edition. 2001. Soil Improvement Committee. California Fertilizer Association. Interstate Publishers. 351 p. (http://agbook.com/ westernfertilizerhb.asp) $35 including shipping.
Plant Nutrition Manual. J. Benton Jones, Jr. 1998. CRC Press, Boca Raton, Florida. 149 p. Approximately $50.
Soil Fertility. Foth and Ellis. 1997. CRC Press, Boca Raton, Florida. 290 p.
Soil Fertility and Fertilizers: An Introduction to Nutrient Management. Havlin, J.L., S.L. Tisdale, J..C. Beaton and W.L. Nelson. 7th edition, 2005. Pearson Prentice Hall. Upper Saddle River, New Jersey. 515 p. approx. $100.
Extension Materials
Fertilizer Guidelines for Montana Crops (EB161), single copy is free. Online at: http://www.montana.edu/ wwwpb/pubs/eb161.html
Soil, Plant and Water Analytical Laboratories for Montana Agriculture (EB 150), single copy is free.
Obtain the above Extension materials (add $1 for shipping) from:
See Web Resources below for online ordering information.
University of Wyoming Fertilizer Recommendations (B1045),$3. Ordering information: Phone: (307) 766-2115.
Personell
Engel, Rick. Associate Professor. Montana State University, Bozeman. (406) 994-5295. gel@montana.edu” engel@montana.edu
Jackson, Grant. Associate Professor. Western Triangle Agricultural Research Center, Conrad. (406) 278-7707. gjackson@montana.edu
Jones, Clain. Extension Soil Fertility Specialist. Montana State University, Bozeman. (406) 994-6076. clainj@montana.edu
Westcott, Mal.

