Soil Sampling and Laboratory Selection
This module is the first in a series of Extension materials designed to provide pertinent information on a variety of nutrient management, water management, and water quality. A resource section of other Extension materials, books, and web sites is also included.
Last Updated: 12/14by Clain Jones, Extension Soil Fertility Specialist; Jeff Jacobsen, former Professor of Soil Science; and Kathrin Olson-Rutz, Research Associate, Department of Land Resources and Environmental Sciences
OBJECTIVES
Soil testing and laboratory selection are critical components of all nutrient management operations. With fertilizer representing a major cost input, it is very important to apply the correct amount of fertilizer and/or soil amendments to optimize economic return. The objectives of this module are to:
- Describe soil sampling and handling procedures,
- Explain the different philosophies in soil testing programs, and
- Provide criteria for selecting analytical laboratories that will provide accurate results.
BACKGROUND
Soil sampling and testing provide an inventory of nutrients in the soil, as well as other soil characteristics that influence soil fertility, such as pH, soil organic matter (SOM) and cation exchange capacity (CEC). Soil testing for plant available nitrogen (N), phosphorus (P), and potassium (K) is done to help determine crop nutrient needs and monitor previous management practices. The other macronutrients (calcium and magnesium) and micronutrients (boron, chlorine, copper, iron, manganese, molybdenum, nickel and zinc) are sometimes analyzed to diagnose nutrient deficiencies. In addition, soil testing is required for concentrated animal feeding operations (CAFO) prior to animal manure application on CAFO fields.
Soil analysis of plant available nutrients is a difficult and sometimes inconsistent process, partly because different analytical procedures may be used to estimate the same plant available nutrient. For example, the “Olsen P” test is generally used in Montana and Wyoming, but labs in the Midwest often use the “Bray” test, largely due to lower soil pH levels in the Midwest. Therefore, the laboratory selected should be familiar with the recommended tests from the states where the soil was collected to use the appropriate analytical procedure. Fertilizer recommendations are subsequently provided by the laboratory or calculated by the end user of the soil test data (see Developing Fertilizer Recommendations for Agriculture; resources mentioned in this bulletin are listed in the appendix). Fertilizer recommendations may vary greatly between laboratories for the same soil, in part due to different fertilization philosophies.
SOIL TESTING
A goal of soil sampling is to characterize the nutrient status of a field as accurately and inexpensively as possible. Details on sampling plans, locations, depths, tools, timing, and sample handling follow.
SAMPLING PLAN
A sampling plan determines where and when to collect soil samples that are representative of the field to be fertilized. If soil is submitted from only a few locations that do not represent the entire area to be fertilized, the fertilizer added may be too much or too little for the majority of the acreage, causing decreased yields, reduced crop quality, or wasted fertilizer.
Sampling depth and timing of sampling are also critical components of a well-designed sampling plan and should be consistent over years. The sampling plan can be constructed in the sampler’s head, but it may be more objective to sketch out the plan ahead of time. The actual locations sampled should be recorded, to provide location-specific fertilization recommendations and to sample same locations in following years.
Sampling Locations
To minimize laboratory costs, soil samples are generally collected from several locations within a field, composited (mixed) in a clean container, subsampled from within that container, and submitted in a bag to an analytical laboratory. The standard sampling method is to collect soils systematically at fixed intervals as shown in Figure 1a. Distance between sampling locations can be greater on homogenous fields than on variable fields.
If more information is desired about the variability within a field, then separate soil samples should be collected from areas that have had different crop history, yield, and fertilizer treatments, or that vary substantially in slope, texture, depth, or soil color (Figure 1b). Either develop specific recommendations for each of the area types, or calculate a field average giving weight to different area samples in proportion to the amount of field area they represent. Furrows, headlands, channels, potholes and straight lines along potential fertilizer bands should be avoided.
To identify sampling zones, start with maps. Soil characteristics are influenced by topography and soil type. Topography maps are used to divide a field into upland, sloping and depression positions which correlate with soil nitrate-N, P, K, zinc, copper, chloride and pH (2,3,4). Maps can be created from actual ground surveys, Light Detection And Ranging (LIDAR), or real-time kinetics-global positioning satellite signals (RTK-GPS).
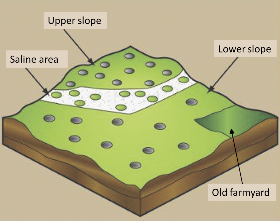
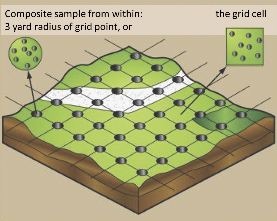
FIGURE 1. Soil sampling schemes in a grid pattern (a) or by zone (b). Adapted from reference 1 and used by permission.
Published soil surveys are of limited value because they are mostly available in an average size delineation of 3 acres (NRCS “Order 2”). This is not detailed enough for site-specific variable rate fertilization (discussed later in this section). Order 1 surveys, with a minimum 1-acre size delineation, are not readily avail- able, are costly, and are not as consistently re- lated to soil nitrate as topography based zones and are no better than 1-acre grid sampling for soil nitrate (5).
Satellite images with 30 meter resolution (¼ acre) are effective in delineating zones. The images are made up of distinct wavelengths which are converted to an index, such as normalized difference vegetation index (NDVI), to represent varying levels of a parameter such as plant nutrient content, water content, soil characteristics (such as color) and yield. If possible, use an image taken in an “average” year at a time between when the canopy closes to just before flowering (see “Web resources” at the end for links to electronic map resources). Because the relationship between indices and any of the above parameters are only estimates based on other research, calculated values should be ground-truthed and verified. Aerial photos can be used but may be hard to get and may be too detailed. Hand-held active optical sensors (e.g., chlorophyll meters) are a good compromise between satellite and aerial photograph resolution and can be attached to equipment used during normal field operations.
Soil electrical conductivity (EC) or soil electromagnetic (EM) sensors have been successfully used to develop nutrient and crop management zones. Both are good at detecting patterns across a field but do not relate to a given soil characteristic. Soil EC has been most useful in coarser-textured soils and fields with salinity problems (4).
Patterns of crop yield are useful to delineate nutrient management zones. Yield maps from a field over several years will indicate zones that are consistently low- or high-producing and can help direct soil sampling. Also, if soil test results indicate adequate or high nutrient levels in low-yielding areas, then the soil should be examined for compaction and other physical characteristics that could affect yield, particularly those that affect water storage or drainage. Fertilizer can likely be reduced in these areas. See Yield Mapping and Use of Yield Map Data for detailed information on yield mapping.
Q&A 1
How do I decide the number of soil samples to collect?
This is dependent on the size of the field, the variability within the field, the fertilizer application equipment, how feasible it is to change application rates within a field, and how much time and money is allocated for sampling. For example, if a producer has 100 acres on a uniform-looking field, all within the same soil series, one composite sample from 20 soil samples is recommended to characterize that field. However, if the field has different depths of a horizon, has visual differences in color or other characteristics, then 20 soil samples may not be adequate. In addition, if the producer has equipment capable of variable rate application, then more samples will allow for more precise application of fertilizer. In a practical sense, the number of cores collected will be largely dependent on the time required to sample and the available sampling budget.
Combining several of these mapping tools to delineate sampling zones is effective in improving soil nutrient management, and increasing yields and quality (6). If a specific factor (such as N) is not a consistent predictor of yield, and yield maps are the primary tool for delineating zones, this may bias the sampling process. The technology used and the resolution obtained in electronic mapping is constantly improving, but most don’t cluster the data into convenient “upland, slope, and depression points.” Compare the map with what you see on the ground.
To determine the worth of sampling by zones, an economic study of continuous corn compared 100-yard sampling grids to sampling by soil series on a 160-acre field (7). Grid sampling produced overall higher yields, but the soil series sampling resulted in greater profits, primarily due to lower analytical costs (6-fold fewer samples) and lower fertilizer costs. General recommendations on when to use grid versus topography sampling are presented in Box 1. For practical reasons, fields are generally broken up into three to five management zones in Montana. Zone based sampling may take more planning time, but it might lead to fewer samples to analyze.
Sampling Number
Table 1 lists the range and average of nutrient concentrations found in a field, and shows why taking a few samples could greatly misrepresent the nutrient status of a field. Generally 12 to 20 sub-samples composited into one sample provide a reasonable representation of a relatively uniform 80-acre field (12, 13). As field size increases, slightly increase the number of sub-samples or submit samples from different zones. Nitrate-N and P are more variable and require more sub- samples for an accurate value than K (Table 2). Fields fertilized with manure, with low nutrient levels, or under conventional tillage require fewer samples. No-till fields and those with fertilizer applied in bands require more sub-samples due to unique distribution patterns of P, K, and pH (13). Soil cores from band rows can contain twice the level of P as cores from between band rows in reduced and no-till fields (14). Banded K also leaves unequal distribution of K, but to a lesser extent than P. If feasible, increase the number of random cores to reduce any bias. With an immobile nutrient like P, look for long-term changes in soil nutrient levels especially in reduced and no- till systems to avoid fertilizing based on “outliers.”
BOX 1. Conditions for using grid vs. zone sampling.
Grid sampling:
- For non-mobile nutrients (e.g., P, K, zinc), especially if previously applied at high levels1.
- History of manure application1.
- High levels of fertilizer previously applied1.
- Small fields merged to form larger fields1.
Zone sampling:
- For mobile nutrients, especially N1.
- For soil organic matter and pH2.
- Unknown field history3 or long cropping history4.
- No history of manure application1.
- History of high within field variability (factor of 2 to 3)
- History of less than maintenance levels of fertilizer application1.
- Remote sensing or yield monitor information indicates relationship of yield to landscape1.
1 - Reference 8; 2 - Reference 9; 3 - Reference 10; 4 - Reference 11
Sampling for site-specific variable rate fertilization, also called “zone management” or “precision agriculture,” generally involves sampling using grids (15). Site-specific yield and grain protein have been combined with soil measurements such as EC, topography, and upslope area to generate models to guide N fertilization and increase net revenue (16). For more information see Site-specific Management Guidelines and What is Site-specific Farming?
Sampling Time
Soil sampling should be timed to allow adequate lead time for sample analysis, data interpretation, fertilizer recommendation, and application, though should be performed as close to seeding as practical. Due to time restrictions or soil conditions, it may be more practical to sample in the late fall or winter for spring-planted crops. Fall sampling results are likely quite similar to spring sampling results if the fall is dry and the winter is cold, restricting microbial activity. Yet, a 3-year Montana study found that nitrate increased about 20 lb N/ acre from late August to early April (17), causing over-fertilization of N on average (since rates in Fertilizer Guidelines for Montana Crops are based on spring sampling results). Coarse or shallow soils (less than 2 feet) have a better chance of overwinter nitrate loss than fine-textured or deep soils, resulting in possible under-fertilization if fertilization rates are based on fall samples (17). Organic matter, pH, and soluble salts are likely to be similar between fall and spring, regardless of weather conditions.
Potassium varies relatively little within a field, but varies greatly over time. It is rapidly released from plant residue and can move quickly among its forms in the soil (18). Therefore, soil test K is influenced by time between harvest and soil sample collection and the weather during that time, which impacts K release from residue. Spring or late fall sampling is likely best. Notes about sampling timing and prior weather can help interpret K soil test values that may seem unusual.
Sampling Depth
The sampling depth should correspond with crop rooting depth. For example, if the bulk of a plant’s roots are located within the top 6 inches of soil, then the relatively immobile nutrients (such as P and K) need only be sampled in the top 6 inches. Sampling depth for the more mobile nutrients (nitrate, sulfate, and chloride) should be to the depth that roots can extract water.
This depth varies with crop, cropping system, and soil depth. For example, winter wheat grown in an alternate crop-fallow cropping system will use water, and therefore access N to approximately 6 feet though sampling that deep is generally unfeasible. However, in a continuous cropping system, winter wheat may only access N to 2 or 3 feet because of the lack of soil water at deeper depths. Therefore, the sample depth for the mobile nutrients is a compromise of usually 2 to 3 feet.
SAMPLING & HANDLING METHODS
Crop residue should be removed from sampling locations. Special areas, such as salty areas, eroded knolls, wet spots, and those without plant growth, should be sampled separately or avoided. Samples should be collected to the maximum depth needed and then divided into depth increments such as 0 to 6 and 6 to 24 inches (Figure 2, page 6). Each depth increment should be mixed thoroughly in a large plastic container, subsampled, and placed into a plastic-lined soil sampling bag or glass jar. Laboratories will either supply sampling bags or can direct you to suppliers.
TABLE 1. Range and average of test values from 40 individual soil cores from an 80-acre field1.
| Analysis | Range (lb/acre) | Average (lb/acre) |
| Nitrate-N | 12-225 | 53 |
| P2O5 | 5-250 | 39 |
| K2O | 156-1164 | 557 |
1 - From reference 12
TABLE 2. Number of sub-samples required to get a test result with a given level of confidence and accuracy, and the range in nutrient level a field could have if sampled for 80% confidence1,2.
| Confidence Level | Accuracy Level | |||||
| ±15% | ±25% | |||||
| Nitrate-N | P2O5 | K2O | Nitrate-N | P2O5 | K2O | |
| Percent | Number of sub-samples | |||||
| 90 | 25 | 34 | 7 | 10 | 12 | 3 |
| 80 | 18 | 21 | 5 | 6 | 8 | 2 |
| 70 | 10 | 14 | 3 | 4 | 5 | 2 |
| Nutrient range (lb/acre) | ||||||
| 80 | 45-61 | 32-45 | 463-626 | 40-66 | 30-68 | 408-680 |
1 - Adapted from reference 12; 2 - Example: 18 samples indicate with 80% confidence that this field has 45-61 lb nitrate-N/acre.

FIGURE 2. Sub-sampling depth intervals.

FIGURE 3. Truck-mounted probe.
Samples should either be kept cool below 40°F, dried at 110 - 120°F, or frozen and dried later to prevent nutrient transformations caused by microorganisms. Soil nitrate levels have been found to increase by 60 to 300% over three days when kept at room temperature and surprisingly by 30 to 200% over three days when refrigerated at 39°F (19). Increases can even occur during drying, likely in the center of larger soil clods which take a while to dry, allowing an extended period of microbial activity; therefore, break up soil clods before drying. Avoid wetting-drying and freezing-thawing cycles. Oven drying above 120°F is not recommended because the excess heat can change the availability of nutrients, making some tests invalid. For example, P can precipitate into relatively unavailable minerals. If drying or cooling equipment is unavailable, samples should be shipped overnight to a laboratory. During any preservation technique, care should be taken to avoid contamination from dust or foreign particles. Sampling equipment (Figure 3) should be cleaned between fields and stored away from fertilizers to prevent contamination.
CHOOSING A LAB
Selecting a laboratory that can supply fast and accurate results can be time-consuming; however, the time spent in choosing a good laboratory can quickly pay for itself in the form of accurate fertilizer recommendations, desired crop responses, and better economic returns. A high degree of variability has been observed in fertilizer recommendations among laboratories. Specifically, some studies have shown that when the same soils were sent to different laboratories requesting a fertilizer recommendation for a specific crop and yield goal, significantly different recommendations have been provided (20, 21, 22). Fertilizer recommendations can differ because of differences in test results and/or the approach used to develop a recommendation.
In general, laboratories’ methods are consistent and provide reliable results for nitrate-N (NO3-N), and K, whereas test results for soil organic matter are least reliable and test results for P are intermediate (23). However, researchers have found substantial differences in soil analytical results that may affect fertilizer recommendations. In a study of 10 laboratories used by northern Great Plains consultants and producers, nitrate-N concentrations reported for the same soil ranged from 3 to 95 pounds N per acre (22). This difference would alter fertilizer recommendations substantially. Phosphorus test results varied from 7 to 42 ppm, and averaged 28 ppm, in one of the four soils tested in this study. Phosphorus fertilizer would likely be recommended for a soil with a 7 ppm test result, yet would likely be wasted on this soil if the actual value was similar to the average soil test P value of 28 ppm (well above the critical level). Selecting a laboratory belonging to a proficiency testing program (discussed below) should greatly improve accuracy.
CROP RESPONSE TO FERTILIZER
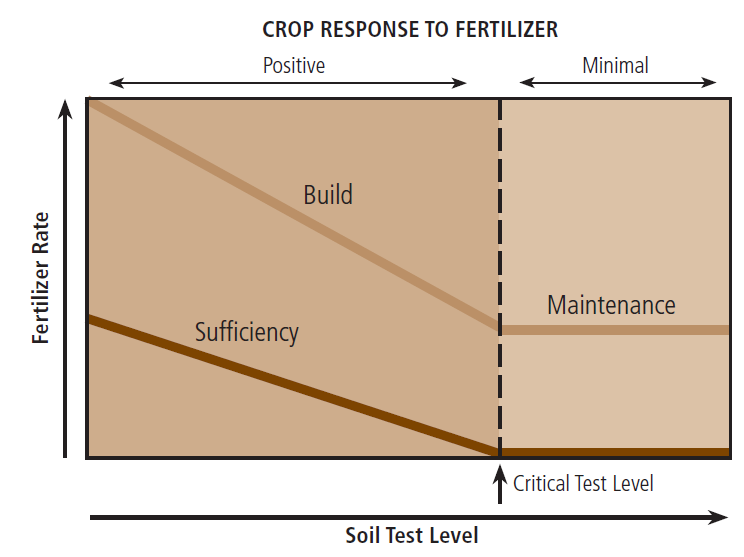
FIGURE 4. Fertilizer recommendation philosophies and crop yield response.
Some of the differences in results may be due to different analytical methods. For example, laboratories either use Bray, Olsen or Mehlich P tests, all of which use different extractants. In Montana’s alkaline soils, P is typically tested using Olsen P, also known as bicarbonate-P. Olsen P is fairly robust and works below pH 7, while Bray does not generally work well above pH 7. Bray and Mehlich test results do not convert readily to Olsen P. Because P fertilizer guidelines for Montana crops are based on Olsen P, ask the soil testing lab to only use this test. Unfortunately, some laboratories do not report which test they use. Therefore it is critical that the user of soil analytical results be aware of the actual analytical procedure since different tests may produce different results.
Fertilizer recommendations are based on a sufficiency, build, or maintenance approach (Figure 4). The sufficiency approach only suggests fertilization when there are insufficient amounts of nutrients in the soil for the present crop. With the sufficiency approach, “critical” values are used to determine whether a field should be fertilized. The critical value is the nutrient test result above which only minimal yield responses are observed when fertilizer is added. A maintenance approach replaces nutrients removed by crop harvest without substantially changing the soil test level, whereas a build approach increases the soil test level over time. The build approach is generally only practiced at low soil test levels, and maintenance is generally only done at higher soil test levels. Table 3 on page 8 demonstrates example P fertilizer recommendations for a sufficiency, maintenance, and build approach at two P soil test levels.
The resulting economic differences between different philosophies and different laboratories can be large. For example, in a multi-year study of fertilizer recommendations from six different laboratories, fertilizer recommendations from one lab were double those of another lab, yet there were no significant differences in yields (24). In another study, the greatest economic return was four times higher than the least economic return based on six different labs’ fertilizer recommendations (20), demonstrating the importance of accurate fertilizer recommendations.
Q&A 2
How do I choose a good soil analytical laboratory?
A list of laboratories in the Northern Great Plains is included in the appendix. Methods used by these labs are described in Interpretation of Soil Test Reports for Agriculture. Start by choosing two or three based on your experience and others’ recommendations. Then, ask questions:
1) Do they participate in the North American Proficiency Testing Program (NAPT) or the Agricultural Laboratory Proficiency Program (ALP)?
2) What other QA/QC protocols (quality assurance/quality control) do they use?
3) Will they send results from both their NAPT or ALP and relevant QA/ QC results?
4) What kind of technical support do they provide? If they don’t know the answers to the above questions, or aren’t willing to send on results, that may be a red flag. If you can obtain results from more than one laboratory, compare the results between laboratories to see which look more accurate (see “Assessing Laboratory Accuracy”).
Growers can assist laboratories in making accurate recommendations by selecting realistic yield goals. These must reflect the soil type and area climate. A recommendation that is satisfactory on calcareous soil in a semi- arid area may be very different than one for a soil containing the same nutrient level but from an area with different soil type and climate.
ASSESSING LABORATORY ACCURACY
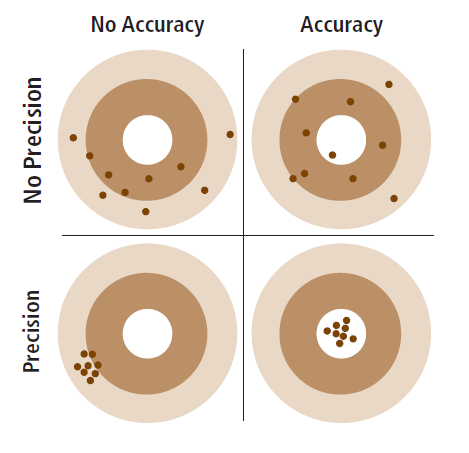
Any quality assurance/ quality control (QA/ QC) data supplied by a laboratory should have both a “true” value and a measured value for each analysis (pH, nitrate-N, P, etc.) for a certified soil, sometimes referred to as an “external standard.” The true values reported are typically the average results from many reputable laboratories for that standard, producing a “mean” value. The Agriculture Laboratory Proficiency Program reports the mean, while the North American Proficiency Testing Program reports the “median” value, which is the concentration that has the same number of labs reporting higher values as lower values, and is often close to the mean. By subtracting the difference between the true and the laboratory measured concentration and dividing this difference by the true value, you can obtain a relative estimate of “error” (accuracy), that is, how close the laboratory’s test comes to the true value (Figure 5). You can compare the errors reported by laboratories that you are considering using, and choose a laboratory that has a low error (high accuracy) for the test(s) that you are most interested in (e.g., nitrate-N, P).
Laboratories should also have high “precision,” which is a measure of how consistent the results are for the same sample (Figure 5). Some laboratories may report the “standard deviation,” which is a measure of how precise the results are (low standard deviation implies high precision).
SUMMARY
Accurate and economical fertilizer recommendations are only as good as the sampling and analytical techniques used to determine these recommendations. A well- designed and implemented soil sampling plan will consider topography, soil type, and sampling timing, depth, and preparation/ storage. The economic worth of each sampling strategy will be dependent on the crop(s) grown, analytical costs, and fertilizer costs. Testing and developing recommendations for specific soils or areas rather than whole fields can produce greater returns if appropriate crop yield goals, accurate soil tests, and reliable fertilizer recommendations are used (25). The experience and knowledge of the land owner or producer should be utilized extensively to select a method of soil sampling. Laboratories range greatly in their accuracy and precision; therefore, laboratories should be selected by comparing their quality assurance/quality control data. Other nutrient management modules address nutrient cycling, testing, and fertilizer recommendations for specific nutrients, such as N, P, and K.
| Recommended P rate (lb P2O5/acre) | ||
| Olsen P soil test 4 ppm | Olsen P soil test 20 ppm | |
| Sufficiency approach1 | 50 | 0 |
| Maintenance approach2 = crop removal | 43 | 43 |
| Build approach = sufficiency + maintenance | 93 | 43 |
1 - Table 18 in Fertilizer Guidelines for Montana Crops; 2 - Table 21 in Fertilizer Guidelines for Montana Crops
FIGURE 5. Accuracy represents how close the samples are to the true value, while precision describes how close the samples are to each other.
REFERENCES
- IPNI, International Plant Nutrition Institute. 2012. 4R Plant Nutrition: A Manual for Improving the Management of Plant Nutrition. International Plant Nutrition Institute, Norcross, Georgia, USA. 66p.
- Franzen, D.W., A.D. Halvorson, and V.L. Hofman. 2000. Spatial nutrient relationships and profitability of variable-rate fertilizer application in a wheat and sunflower rotation. p. 88-93. In: Great Plains Soil Fertility Conference Proceedings. p. 88-93. March 4-8. Denver, Colorado. A. Schlegel [Ed.]. Potash & Phosphate Institute, Brookings, South Dakota.
- Franzen, D.W., T. Nanna, and W.A. Norvell. 2006. A survey of soil attributes in North Dakota by landscape position. Agronomy Journal. 98:1015-1022.
- Franzen, D., 2013. Developing Zone Soil Sampling Maps. North Dakota State University Extension Publication SF-1176-2. 4p.
- Franzen, D.W., D.H. Hopkins, M.D. Sweeney, M.K. Ullmer, and A.D. Halvorson. 2002. Evaluation of soil survey scale for zone development for site-specific nitrogen management. Agronomy Journal. 93:381-389.
- Franzen, D., T. Nanna, F. Casey, D. Ralston, J. Staricka, M. Halvorson, V. Hofman, J. Lamb, and A. Sims. 2005. Zone delineation for nitrogen management. 21:25-32. November16-17. Des Moines, Iowa. Potash & Phosphate Institute, Brookings, South Dakota.
- Clay, D.E., J. Chang, C.G. Carlson, D. Malo, S.A. Clay, and M. Ellsbury. 2000. Precision farming protocols. Part 2. Comparison of sampling approaches for precision phosphorus management. Communications in Soil Science and Plant Analysis. 31:2969-2985.
- Franzen, D.W., L.J. Cihacek, V.L. Hofman, and L.J. Swenson. 1998. Topography-based sampling compared with grid sampling in the Northern Great Plains. Journal of Production Agriculture. 11:364-370.
- Mallarino, A.P. and D.J. Wittry. 2004. Efficacy of grid and zone soil sampling approaches for site-specific assessment of phosphorus, potassium, pH, and organic matter. Precision Agriculture. 5:131-144.
- Franzen, D. Personal communication. Extension Soil Specialist, Department of Soil Science, North Dakota State University. Fargo, North Dakota.
- Mallarino, A.P., D.B. Beegle, and B.C. Joern. 2006. Soil Sampling Methods for Phosphorus – Spatial Concerns. A SERA-17 Position Paper.
- Swenson, L.J., W.C. Dahnke, and D.D. Patterson. 1984. Sampling for Soil Testing. North Dakota State University, Department of Soil Sciences, Research Report No. 8.
- Miller, R. 2011. Grid point soil sampling phosphorus and potassium uncertainty. NCERA 13 Workshop Proceedings. p. 60-61. February 22-23. Bettendorf, Iowa.
- Mallarino, A.P., and R. Borges. 2006. Phosphorus and potassium distribution in soil following long-term deep- band fertilization in different tillage systems. Soil Science Society of America Journal. 70:702-707.
- Franzen, D.W. and T.R. Peck. 1995. Field soil sampling density for variable rate fertilization. Journal of Production Agriculture. 8:568-574.
- Barroso, J., P. Lawrence, C. Merja, and B. Maxwell. 2014. Spatial Optimization of Nitrogen Application for Wheat. Fertilizer Facts No. 65. Montana State University. 2p.
- Jones, C., C. Chen, J. Eckhoff, M. Greenwood, P. Lamb, A. Lenssen, K. McVay, P. Miller, B. Stougaard, J. Weeding, and M. Westcott. 2011. Changes in soil nitrate-N levels from late summer to early spring in Montana. Fertilizer Facts No. 55. Montana State University. 2p.
- Mallarino, A.P., P.A. Barbagelata, M.W. Clover, C.X. Villavicencio, and L.B. Thompson. 2011. Factors determining high temporal soil-test potassium variation and soil sampling and testing alternatives. NCERA 13 Workshop Proceedings. p. 3-11. February 22-23. Bettendorf, Iowa.
- Westfall, D.G., M.A. Henson, and E.P. Evans. 1978. The effect of soil sampling between collection and drying on nitrate concentration. Communications in Soil Science and Plant Analysis. 9:169-185.
- Davis, J.G., D.B. Bosley, R. Buhler, A.W. Cooley, T. Macklin, R.F. Meyer, F. Sobolik; and K.V. Iversen. 1999. Comparison of soil testing laboratories. In Proceedings of the Western Nutrient Management Conference. 3:4-5. March 4-5. Salt Lake City, Utah. Potash & Phosphate Institute, Brookings, South Dakota.
- Follett, R.H., and D.G. Westfall. 1986. A procedure for conducting fertilizer recommendation comparison studies. Journal of Agronomic Education. 15:27-29.
- Jacobsen, J.S., S.H. Lorbeer, B.E. Schaff, and C.A. Jones. 2002. Variation in soil fertility test results from selected Northern Great Plains laboratories. Communications in Soil Science and Plant Analysis. 33:303-319.
- Miller, R. 2013. Reliability of soil and plant analyses for making nutrient recommendations. Western Nutrient Management Conference. March 7-8, 2013. Reno, Nevada.
- Follett, R.H., D.G.Westfall, T.J. Doherty, E.E. Rothman, E.J. Langin, and H.M. Golis. 1984. Soil Test Recommendation Studies. Service in Action No. 511. Colorado State University, Extension Service, Fort Collins, Colorado, 1984.
- Carr, P.M., G.R. Carlson, J.S. Jacobsen, G.A. Nielsen, and E.O. Skogley. 1991. Farming soils, not fields: a strategy for increasing fertilizer profitability. Journal of Production Agriculture. 4:57-61.
APPENDIX
EXTENSION MATERIALS
Developing Fertilizer Recommendations for Agriculture. Montana State University Extension Publication MT200703AG
Fertilizer Guidelines for Montana Crops. Montana State University Extension Publication EB0161
Interpretation of Soil Test Reports for Agriculture. Montana State University Extension Publication MT200702AG
Site-Specific Management Guidelines. IPNI, International Plant Nutrition Institute. http://www.ipni.net/ssmg
What is Site-specific Farming? North Dakota State University Extension Publication SF-1176-1
Yield Mapping and Use of Yield Map Data. North Dakota State University Extension Publication SF-1176-3
Obtain the MSU Extension materials from:
MEDIA
4R Nutrient Stewardship – Overview. 2012. International Plant Nutrition Institute. CD. www.ipni.net
4R Plant Nutrition. 2012. International Plant Nutrition Institute. Book and CD. www.ipni.net
Plant Nutrition and Soil Fertility Manual. 2nd ed. 2013. J. Benton Jones, Jr., CRC Press/Taylor & Francis Group, Boca Raton, Florida. 304 p.
Site-Specific Management Guidelines. 2014. International Plant Nutrition Institute. A publication series on site-specific soil and crop management issues. www. ipni.net/ssmg
Soil Fertility Manual. 2003. International Plant Nutrition Institute. 200 p. Book or CD. www.ipni.net
Soil Science Step-by-Step Field Analysis. 2008. S. Logsdon, D.Clay, D. Moore, and T. Tsegaye [Eds.]. Soil Science Society of America. www.soils.org
Soil Testing: Prospects for Improving Nutrient Recommendations. 1994. J.L. Havlin and J.S. Jacobsen. Soil Science Society of America Special Publication No. 40. 216 p. https://dl.sciencesocieties.org/publications/ books/tocs/sssaspecialpubl/soiltestingpros
Stewardship Specifics. 2013. International Plant Nutrition Institute. One page summaries of issues impacted by nutrient stewardship. www.ipni.net
Western Fertilizer Handbook. 9th ed. 2002. Western Plant Health Association. Waveland Press. 356 p.
WEB RESOURCES
http://landresources.montana.edu/soilfertility/ Clain Jones’ Extension Soil Fertility webpage with presentations, publications and links to information.
http://landresources.montana.edu/fertilizerfacts/ Fertilizer findings and recommendations based on MSU field research.
http://landresources.montana.edu/nm/ Nutrient Management modules covering assorted soil nutrient topics.
http://landresources.montana.edu/swm/ Soil and Water Management modules covering assorted soil and water topics..
http://geoinfo.msl.mt.gov/Home/data/ aerial_photographs_of_Montana.aspx Geographic Information Clearinghouse at the Montana State Library provides National Agricultural Imagery Program (NAIP) images to use with GIS or other image service viewers (http://gisservice.mt.gov/arcgis/rest/services/MSDI_Framework >MSDI_Framework/NAIP_2011/ImageServer).
http://datagateway.nrcs.usda.gov/ Geospatial Data Gateway provides the Web Soil Survey (http://websoilsurvey.sc.egov.usda.gov/), National Agricultural Imagery Program (NAIP; www.fsa.usda.gov/FSA/ apfoapp?area=home&subject=prog&topic=nai) aerial imagery, and other products.
http://nationalmap.gov/ The National Map managed by USGS National Geospatial Program.
http://ned.usgs.gov/ National Elevation Dataset (NED) feeds elevation data to The National Map project
www.usgs.gov/pubprod/index.html USGS maps, imagery and publications.
www.lidarbasemaps.org/ LIDAR Links for Mappers lists links to LIDAR map sources.
LABORATORY PROFICIENCY PROGRAMS
Agricultural Laboratory Proficiency Program (ALP), www.collaborativetesting.com/store/main.aspx?DepartmentId=40
North American Proficiency Testing Program (NAPT), www.naptprogram.org/
SOIL, PLANT AND WATER ANALYTICAL LABS FOR MONTANA AGRICULTURE
(Italicized give recommendations based on Montana guidelines.)
Manure Analysis Proficiency (MAP) labs are listed by MN Dept. of Ag at www2.mda.state.mn.us/webapp/lis/maplabs.jsp
Note: There are likely other laboratories in the Northern Great Plains that can meet your analytical needs.
QUESTIONS?
Contact Clain Jones, MSU Soil Fertility Extension Specialist, clainj@montana.edu, 406-994-6076.
ACKNOWLEDGMENTS
We would like to extend our utmost appreciation to the following who provided their time and insight to make this a better document:
- Paul Dixon, former Yellowstone County Extension Agent, Billings, MT
- Chuck Gatzemeier, CG Ag Consulting, Cut Bank, MT
- Grant Jackson, retired from Western Triangle Agricultural Research Center, Conrad, MT
- Dan Lucas, Granite County Extension Agent, Philipsburg, MT
- MSU Extension Communications & Publications for design and layout

