
Forage Nitrate Analysis: What Method to Use?
Utilizing tests to determine nitrate accumulation in forage is an important tool for producers. This publication outlines the protocols and efficacy of the Nitrate QuikTest, Nitrate Strip Test, and Commercial Laboratory Analysis.
Last Updated: 05/19by Emily Meccage, PhD, MSU Extension Forage Specialist; Zach Miller, PhD, Superintendent, Western Agricultural Research Center, MSU; and Danielle Peterson, MS, MSU Forage Research Lab Manager
ALL RANCHES RELY ON FORAGES SUCH AS
native range, introduced pasture, or hay to feed their livestock. Many livestock enterprises in Montana use a combination of several types of forages, including some that have the potential to contain toxic levels of nitrates (NO3-). Nitrate toxicity associated with feeding forages reduces an animal’s ability to transport oxygen in the blood, especially young livestock, and can have major implications on livestock production. Animals subject to chronic NO3- toxicity due to sustained moderate levels of NO3- in feed and/or water exhibit reductions in appetite, reproduction, and productivity. Acute NO3- toxicity, which is characterized by animals consuming forages with toxic levels of NO3- in a short amount of time, can be fatal. Ruminants like cattle, sheep, and goats are more prone to NO3- toxicity than non-ruminants such as horses and pigs.
Annual cereal forages, such as wheat, barley, and oats, are prone to accumulating NO3- that can harm livestock and ranch profits. Other species, including some grasses, sorghum, corn, brassicas, millet, sweet clover, alfalfa and weeds, such as kochia, lambsquarter, and pigweed, can also accumulate NO3-. Beyond species, there are many factors that are implicated in toxicity, including: environment, water availability, forage maturity, herbicide, and fertilizer use, among others. This document will discuss various methods of testing forages for potential NO3- toxicity. For more information regarding NO3- toxicity in animals, please refer to the MSU Extension MontGuide Nitrate Toxicity of Montana Forages (MT200205AG).
Recommended Nitrate Levels
Nitrate levels in plants fluctuate depending on several factors: environmental conditions such as drought, hail, or frost; producer management techniques, including manure or fertilizer application; time of day at harvest; and forage species. Variation in plant NO3- levels has led to the development of field tests that can estimate NO3- levels in forage. These tests can be used prior to harvest to help producers reduce the risk of harvesting and feeding forages that contain toxic levels of NO3-; this is particularly important as levels do not decrease after harvest. Nitrate tests are also valuable for use post-harvest to adjust feed rations based on NO3- levels. Additionally, some of these tests can be used to test for NO3- in water. Water can have high NO3- content in some wells and ditches, which can be a significant NO3- source to livestock. It is important to account for both feed and water NO3- content when feeding forages to livestock. Recommended NO3- levels while feeding livestock are described in Table 1.
TABLE 1. Recommended Nitrate Levels for Feeding Livestock, from Nitrate Toxicity of Montana Forages, MT200205AG
| Nitrate (ppm NO3-) | <1,500 | 1,500 - 5,000 | 5,000 - 10,000 | >10,000 |
| Recommendation for feeding | Generally considered safe for all livestock | Limit to 50% for calves, pregnant, or lactating animals. | Limit to 25-50% feed. Do not feed to pregnant animals. | Do not feed as is. Can cause animal mortalities. |
Testing Methods
The ability to rapidly and accurately test NO3- levels in annual forages is important for producers in Montana. Currently, many producers test NO3- levels using the Nitrate QuikTest (Figure 1). The Nitrate QuikTest is a qualitative method developed in the 1960s that detects the presence of NO3- with a change in color of the testing solution, which consists of diphenylamine in 82% sulfuric acid. This test must be handled with caution and can only be accessed by trained and certified personnel, such as your local Extension Agent. The test is administered by first splitting the stem of a plant longitudinally and then placing 1-2 drops of QuikTest solution onto the lower nodes, where plants tend to accumulate the highest levels of NO3-. If the plant contains a detectable amount of NO3-, the solution will turn dark blue or purple in color (Figure 2). While the test is simple to use, it does not provide a clear, quantitative measure of NO3- level, which is needed to determine whether a forage is safe to feed to livestock (Table 1).
The most accurate method for quantitative NO3- detection is laboratory analysis. Samples that are sent to the lab are tested using wet chemistry methods that have been approved by the Association of Official Analytical Chemists. Table 2 (page 3) is a laboratory analysis of barley hay. The analysis in Table 2 reports NO3- as a percentage; however, each lab is different and may choose to report nitrate levels as parts per million (ppm) NO3-, ppm nitrate as nitrogen (NO3-N), or as a percentage. Conversion factors can be found in the MontGuide Nitrate Toxicity of Montana Forages (MT200205AG).
Forages vary in moisture contents, so it is important to use dry weight/dry matter NO3- values when developing rations to feed to livestock. This allows comparisons to be made between forages of different moisture content and reflects actual amounts of feed ingredients excluding water content, which can dilute ingredient values. In Table 2, the NO3- content is 0.46% on a dry weight basis, which is equivalent to 4600 ppm NO3- (% NO3- × 10,000 = ppm). Based on the results of this lab analysis, consumption of this barley hay must be limited to below 50% of feed ration for calves and pregnant or lactating animals (Table 1).
Although laboratory analysis is the most reliable method available, it is also time consuming and costly, with a turn-around time from two days to several weeks. For this reason, other quantitative NO3- detection tests, such as the Nitrate Strip Test (Figure 3, page 3), are being considered. The Nitrate Strip Test consists of a reducing agent, an acidic buffer, and chemical compounds that interact and produce a red-violet dye. When a reaction occurs, NO3- levels can be measured semi-quantitatively by visually comparing the reaction zone of the test strip (Figure 4, page 3) to a color scale (Figure 3) representing different categories of NO3- levels.
Figure 1. Nitrate QuikTest
Figure 2. Left: Plant stem prior to QuikTest. Right: Plant stem after QuikTest. The dark blue color indicates the presence of nitrate in this plant.


TABLE 2. Laboratory analysis of barley hay in Montana
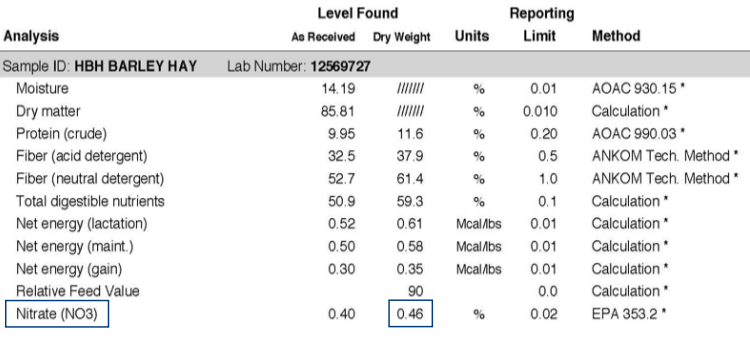
FIGURE 3. Nitrate Strip Test

FIGURE 4. Strips that have been used to test plants for nitrates

Table 3. Nitrate Strip Test Reading and Corresponding Forage Nitrate Content
|
Test Strip Reading
(mg/L NO3)
|
Forage Nitrate
(mg/kg NO3)
|
| 0 | <1,000 |
| 10 | 1,000 |
| 25 | 2,500 |
| 50 | 5,000 |
| 100 | 10,000 |
| 250 | 25,000 |
| 500 | 50,000 |
Instructions for the Nitrate Strip Test vary depending on the brand selected for use. Generally, forages must be dried and ground prior to testing. These two steps can be done at home using a microwave to dry the forage and either a blender or a coffee grinder to grind the forage. After collecting a fresh forage sample, cut the sample into 1-2 inch lengths using scissors. Spread the cut samples in a single layer onto a microwave dish and microwave on high setting using 30-second intervals or until dry. Be careful not to heat the samples rapidly, or for extended periods of time, to avoid charring. Place the dried sample into a coffee grinder or blender and grind until the particle size of the dried forage resembles that of salt or sugar. Then, combine the ground sample with low NO3- tap water or distilled water and allow it to soak for about 30 minutes, usually at a ratio of 100 parts water to 1 part forage. Always refer to your protocol for correct amounts. After 30 minutes, dip the Nitrate Strip Test strip into the forage-water mixture for 2 seconds, remove, and allow to react for 1 minute. After 1 minute, the strip can be compared to the color scale provided on the testing kit to determine the semi-quantitative NO3- concentration in the forage being tested (Figures 3 and 4; Table 3).
Regardless of the testing method used to etermine NO3- in forage, it is important to obtain representative sample for analysis. Samples are considered adequate for analysis when they represent the variations within a pasture or hay stack as possible. The best way to achieve an adequate sample is to walk in a zig-zag pattern and select clippings randomly throughout the pasture, or to sample several bales throughout the stack. The number of samples should increase with increasing pasture size or number of ales to achieve a representative sample for the entire pasture. Collected samples should be mixed and either dried, or frozen, prior to shipping to the lab. For more information on collecting a representative forage feed sample for analysis refer to the MontGuide Collecting a Forage or Feed Sample for Analysis (MT201610AG).
Montana Research
In order to provide more accurate and expedited results to producers, the two quantitative methods for Nitrate detection, the Nitrate QuikTest and the Nitrate Strip Test, were compared to a Nitrate Laboratory Analysis in order to determine test accuracy. See Table 4 for a description of all three analyses. Laboratory analysis is considered to be the “gold standard” for testing because it is the most accurate method for NO3- determination. Lab analysis directly measures the NO3- concentration contained within the sample via wet chemistry methods. For this reason, both the Strip Test and QuikTest are compared to lab analysis to determine accuracy.
FIGURE 5. Compares the results of samples analyzed using the Strip Test and commercial lab analysis for various forages grown in Montana.
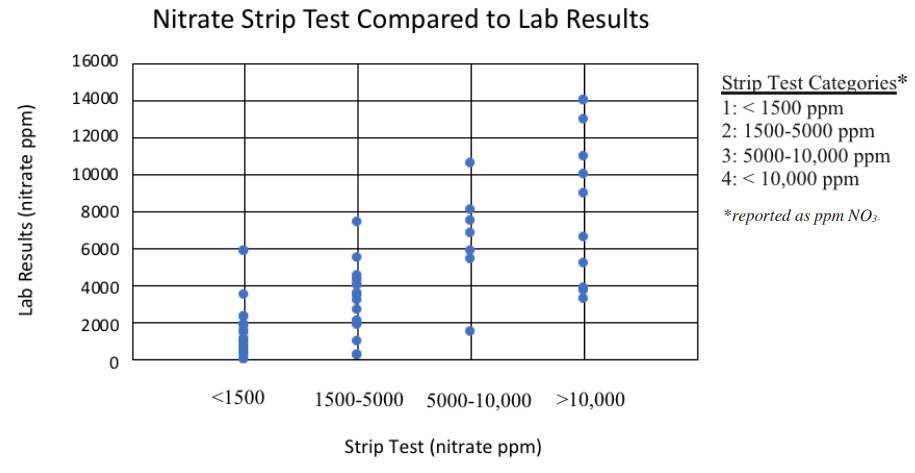
FIGURE 6. Compares results from the Nitrate QuikTest with commercial lab analysis for various forages grown in Montana. Yes using the QuikTest indicates a positive result, and the presence of nitrates, while No indicates a negative result, and lack of nitrates present.
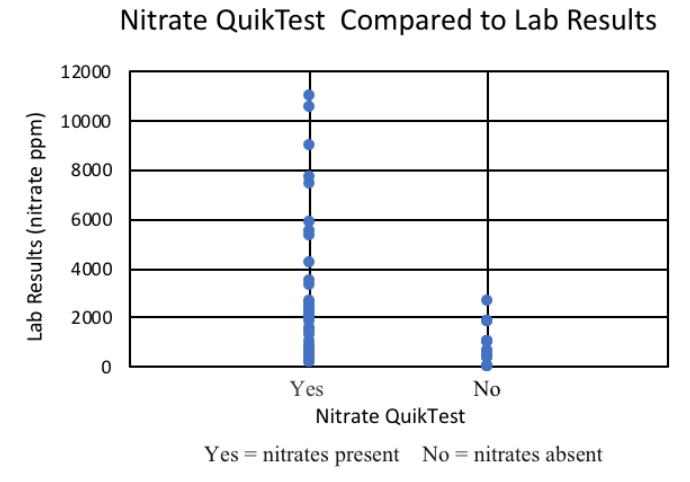
TABLE 5. A comparison of the accuracy of results of the Strip Test and the QuikTest compared to the laboratory analysis. A false positive means the individual test has higher levels of NO3- than the laboratory analysis. A false negative means the individual test has lower NO3- levels than the laboratory analysis.
| Strip Test | QuikTest | ||
|
Sample Number
(Percent of Total Samples)
|
|||
| Correct | 54 (71%) | 45 (71%) | |
| Incorrect | False + | 10 (13%) | 15 (23%) |
| False - | 12 (16%) | 4 (6%) | |
| Total | 76 | 64 | |
A two-year study in Montana, conducted by Meccage et al., evaluated NO3- levels using both the QuikTest and Strip Test, and compared the results to lab analyses. The purpose of the study was to determine the validity of using the Strip Test and QuikTest to detect NO3- in a production setting, and to evaluate the accuracy of the Strip Test in detecting quantitative NO3- levels. Data for the study was compiled using samples taken by Montana State University Extension agents across the state. The data consisted of NO3- detection through the use of the Nitrate QuikTest, Nitrate Strip Test, and commercial laboratory analysis for 16 forage classes in 14 counties.
Effectiveness of Individual Tests
Strip Test
The Strip Test offers producers the opportunity to obtain a semi-quantitative measure of NO3- accumulation in forage. Nitrate results were then placed into four different categories: Category 1: <1500, Category 2: 1500-5000, Category 3: 5000-10,000, and Category 4: >10,000 ppm NO3- (Figure 5, page 5). This study found that the Strip Test was a reasonably reliable method for quantitative NO3- detection, with an accurate result compared to the commercial analysis 71% of the time (Table 5, page 5). Of the 29% inaccurate estimates, 45% overestimated NO3- levels compared to the commercial lab analysis and the remaining 55% underestimated NO3- levels compared to the commercial lab analysis. When evaluating the incorrect results, about 73% of them were only off in estimation by one category, but 6 of the 22 incorrect samples were off by at least 2 categories. It is also important to note, that when the strip test yielded a false negative, the results were still always below 5,000 ppm, meaning they would have still been considered “safe” for non-pregnant animals. Caution should be used when feeding pregnant animals, but for open livestock, this test could be a reliable option.
Based on these results, it is recommended that producers are cautious when using the Strip Test to detect quantitative NO3- values because it was only accurate 71% of the time. To minimize the chance of achieving inaccurate results, it is especially important to obtain a representative sample, and samples that test anywhere near 1500 ppm for NO3- need to be sent to a commercial laboratory for analysis prior to being fed to livestock.
QuikTest
The Nitrate QuikTest offers producers the opportunity to obtain a qualitative measure of NO3- accumulation in forage, and was found to be similar in accuracy compared to the Strip Test. In this study, the QuikTest reported correct results 71% of the time compared to commercial quantitative analysis (Table 5). Of the total samples, 23% of the samples were false positives, while only 6% were false negatives. This is in accordance with reports from producers who have stated that they commonly have to delay harvest due to QuikTest results, but receive “safe” results after submitting for commercial analysis.
It should be pointed out that of the 4 false negatives, 3 of those samples had a nitrate level around 1900 ppm, which while incorrect and reported as a false negative, is quite close to the recommended threshold safe level for feeding. It is also worth noting that even with the false negative samples reported by the QuikTest, all of those samples were still below 5,000 ppm, and would have been safe to feed to non- pregnant livestock, similar to the Strip Test.
Summary
Nitrates in plants fed as processed feed, and as forage, are a concern for livestock producers. Quick, accurate methods of quantitative NO3- detection are crucial to minimize delays in harvest and for turning animals onto NO3- containing forages. The Nitrate QuikTest and Nitrate Strip Test are two cost-effective methods in which producers can quickly and easily test for the presence of NO3-. The Nitrate QuikTest is qualitative and is only useful in detecting the presence of NO3- in plants. The Nitrate Strip Test is semi-quantitative and is limited to categorizing plants into a general range of NO3- content.
Both of these tests are useful for preliminary NO3- detection; however, research indicates that these tests can give misleading and inaccurate results. This research indicates that both of the tests are similar in accuracy, correctly estimating nitrate levels about 70% of the time when compared to a commercial analysis. Of the inaccuracies, the Strip Test was almost equal in reporting false positives versus false negatives, while the QuikTest reported false positives much more commonly. These can both provide a reasonable estimate of assessing nitrate risk, but they cannot be relied upon to give accurate results 100% of the time. There is a benefit to using the Strip Test in that it is a semi-quantitative estimate, and many of the samples that were marked as incorrect still actually detected levels of nitrate, they just did not indicate the appropriate category, or the interpretation of the color change was incorrect. The benefit of the QuikTest is that it much more commonly provided false positives compared to false negatives, decreasing risk of feeding unsafe feedstuffs, however both tests still have potential for inaccurate interpretation.
Caution must be exhibited when utilizing these two tests to detect NO3- in forages that are meant to be consumed by livestock. Laboratory analysis is still the most reliable method for quantitative NO3- detection, and although sending samples to the lab is the most time consuming, it is the only way to obtain the actual numerical NO3- content of a plant. The most important aspect of sampling for NO3-, regardless of the detection test being used, is obtaining a representative sample. Failure to use a representative sample for analysis will increase the chance of inaccurate results and can be dangerous for livestock.
Resources
Commercial Laboratory Analysis
Information regarding commercial laboratories can be found by contacting a local Extension agent, Extension Forage Specialist, Certified Crop Adviser, or through individual laboratory websites. A complete list of certified laboratories can be found on the MSU Extension Forages Website. Generally, lab protocol will require producers to acquire a representative sample to submit for analysis.
Nitrate QuikTest
The Nitrate QuikTest is only available for use by trained and certified personnel. Contact your local Extension agent to access this test or to become certified to use this test.
Nitrate Strip Test
The Nitrate QuikTest is only available for use by trained and certified personnel. Contact your local Extension agent to access this test or to become certified to use this test.
Terminology for this study
False negative: results that indicated lower levels of nitrates using the qualitative test compared to quantitative testing. For the QuikTest, this means there were no color changes, but were positive quantitative results. For the Strip Test, this means that the test estimated the nitrates were in a lower category than the quantitative test.
For example, the Strip Test may indicate that a sample was category 2 (1500-5000ppm NO3-), but if the quantitative estimated it was >5000ppm NO3-, it was marked negative.
False positive: results that indicated higher levels of nitrates using the qualitative tests compared to the quantitative analysis. For the QuikTest, this means that there was a color change but no elevated levels. (<5000ppm NO3-) on the quantitative test. For the strip test, this means the test rated the risk as higher than it was.
For example, the Strip Test may indicate that a sample was in a category 3 (5,000-10,000ppm NO3-), but it may have actually only been in category 1 or 2 (<5,000ppm NO3-) based on the quantitative test.
*The means reported in this publication are least squares means. Least squares means are means that have been adjusted for other terms in a model (county, year, treatment).

