
Fungicide Use in Field Crops: Classification, Risks, Use & Economics
Fungicides are effective tools for disease management. This Montguide covers when fungicides could be used, what types of fungicides are available, the risk of fungicide resistance, where to find data on fungicide efficacy, and the economics of fungicide use in wheat.
Last Updated: 07/17by Mary Burrows, Ph.D., Extension Plant Pathologist; Kate Fuller, Ph.D. Extension Economics Specialist; and Jessica Rupp, Ph.D., Extension Plant Pathologist
PLANT DISEASES ARE CAUSED BY MANY DIFFERENT
organisms including fungi, bacteria, viruses, nematodes, and others. Fungicides are pesticides used for controlling fungal and fungal-like diseases and can be delivered as a seed treatment, in-furrow application or foliar spray. Seed and in-furrow fungicide treatments target seed- and soil-borne fungal diseases, while foliar applications target mainly leaf and stem fungal diseases. Fungicides work, in general, by blocking a specific metabolic pathway in the fungus that prevents spore germination or hyphal growth. These different mechanisms are called modes of action.
Before deciding to apply a fungicide, ask several questions:
- Are the symptoms observed due to a fungal disease?
- Is the fungicide effective on the plant disease of concern?
- Do the economics of the system justify the application?
1. Are the symptoms I’m observing due to a fungal disease?
Plant diseases can be difficult to diagnose. Symptoms of plant diseases are often confused with nutrient deficiency and other abiotic factors including mechanical damage to the plant (hail, wind, insect feeding, herbicide injury, etc.), and temperature stress (hot, cold). Plant disease diagnosis is easier with experience, but comes down to a combination of familiarity with symptoms and look-alike symptoms, an investigation of the pattern and timing of symptom appearance, and, when needed, testing for the pathogen of interest for confirmation.
If a particular disease is suspected, look up the disease cycle and determine what the source of inoculum is and the description of symptoms, and decide whether these fit the observed symptoms. For example, foliar diseases of wheat other than cereal rusts are generally residue-borne - carried on plant materials left over from the previous crop. Are the symptoms observed highly associated with residue (lower- and mid-canopy leaves affected first)? Bacterial diseases can be highly associated with hail or wind events that wound plant tissue. Viral diseases need a vector to transmit them to the plant, and patterns of diseased plants (clustered/aggregated) will match vector (i.e. insects) movement into the field.
Plant disease only occurs when the pathogen is present, the host is susceptible to infection, and the environment is favorable for disease development. This is commonly known as the disease triangle. There are many sources to find information about the disease cycle for a pathogen, information about host susceptibility, and environmental conditions favoring disease development.
2. Is the fungicide I’m considering using effective on the plant disease I’m concerned with?
After identifying the plant disease you are concerned with and confirming that it is fungal, treatment options can include fungicides. However, not all fungicides are effective against all fungi. There is also a range of efficacy of products against fungal diseases. Consider the mode of action of the fungicide, get information from other sources about the efficacy of the product you are considering to use, and determine rate, method, and number of applications that may be required for disease management.
Fungal modes of action
Fungicides can be either non-systemic (contact) or systemic. Systemic movement by a fungicide is not the same as systemic movement by an herbicide. Fungicides that are non-systemic remain on the plant surface and do not enter the plant. Systemic fungicides are able to enter and move to various degrees throughout plant tissue. Fungicides are classified by their mode of action, and these modes of action (MOA) are then classified according to the possibility of fungal pathogens quickly becoming resistant (i.e. fungicide no longer controls the fungal pathogen). The Fungicide Resistance Action Committee (FRAC, www.frac.info) is tasked with classifying fungicides into different MOAs. A list of resistant organisms can be found on the FRAC website (www.frac.info). It is important to avoid the development of fungicide resistance as described below. There are four main classes of systemic modes of action (e.g. Group 1, 3, 4 and 7). Databases for identifying what products are registered on a crop are available from CDMS (www.cdms.net), Greenbook (www.greenbook.net), the North Dakota State University fungicide guide (www.ag.ndsu.edu/publications/), the Pacific Northwest Plant Disease Management Guidebook (http://pnwhandbooks.org/plantdisease/) and other sources. A list of products approved for organic production can be found at the Organic Materials Review Institute (OMRI, www.omri.org).
FRAC group 1: MBC fungicides, Methyl Benzimidazole Carbamates
Active ingredients and common/trade names: thiabendazole (Mertect-340F), thiophanate-methyl (Topsin)
Residual on plant tissue: 14-28 days
Risk of fungicide resistance development: High
Mobility in plant: acropetal penetrant, xylem mobile
Protection of plant tissues: protects entry point and younger tissue
Mode of action: interfere with cell division - mitosis: broad spectrum
Benzimidazoles were first introduced on the market in the 60s and 70s. They have low use rates, broad-spectrum activity, systemic movement, and post-infection activity that allowed for greater intervals between applications. Resistance development with these products is rapid due to the single site mode of action.
FRAC group 3: DMI, Demethylation Inhibitors
Active ingredients and common/trade names: imazalil (included in Raxil MD-Extra W); propiconazole (Tilt); prothioconazole (Proline); tebuconazole (Folicur); metconazole (Caramba, Quash); also available as blends with other MOAS
Residual on plant tissue: Depends on the specific product; 10-30 days
Risk of fungicide resistance development: Medium
Mobility in plant: High systemic activity
Protection of plant tissues: Protective and curative
Mode of action: interfere with biosynthesis of sterols in fungal cell membrane; spore penetration and mycelial growth.
Demethylation inhibitors (DMI) fungicides, also called sterol biosynthesis inhibitors, were first introduced in the 70s. These products were a major breakthrough: they provide curative and protective activity at low application rates and have a high degree of systemic movement in the plant. They inhibit the formation of sterols, which are required for fungal cell wall formation, and thus are effective at preventing hyphal growth. The largest group of DMI fungicides, the triazoles, are commonly used on grain crops as curative and preventative products. They are particularly active against most rusts and have good preventative activity for Fusarium head blight on grains. They are also effective against powdery mildew and many foliar blights. Efficacy varies by active ingredient and disease combination. Mobility in the plant is much higher than the group 11 (QoI) fungicides: they are translaminar (move across leaf surfaces) and are transported by the transpiration stream (xylem, not phloem). Thus, they are active on the leaf to which they have been applied and new growing portions of the plant. Some may have limited downward movement in the plant (a few millimeters). Fungicides applied to leaves are highly unlikely to move (translocate) to the roots. Chemical that runs down between leaf sheaths may be transported into buds and possibly stems. Residual time and mobility varies by active ingredient. DMIs can have growth regulator effects, causing shortened internodes and smaller, greener leaves.
Resistance development is quantitative since there are multiple sites that need to be mutated in order to confer complete resistance. Dose of the fungicide will increase over time as the pathogen is exposed to DMI fungicides, but complete failures in a short time span are not as likely. Repeated use of these compounds has led to resistance in diseases such as powdery mildew of grape and apple scab. This is of particular risk on diseases that cycle quickly during the season and require multiple applications. Cross-resistance is possible.
FRAC group 4: Phenylamide fungicides
Active ingredients and common/trade names: metalaxyl (Apron), mefenoxam (Allegiance)
Residual on plant tissue: 2-3 weeks
Risk of fungicide resistance development: High
Mobility in plant: acropetal penetrant, xylem-mobile systemic movement from roots to shoots
Protection of plant tissues: Protective and suppressive for oomycetes (water molds).
Mode of action: Inhibits RNA synthesis, suppresses sporangial formation, mycelial growth, and establishment of new infections; does not inhibit zoospore release, zoospore encystment (a dormant stage), or initial penetration of the host. Phenylamide fungicides are active exclusively on oomycetes including Pythium, Phytophthora, and downy mildew pathogens. They were first introduced on the market in 1977 and were very successful due to properties including high potency, curative and protective activity, excellent redistribution and protection of new plant growth, control of all oomycetes, and flexible application methods including seed treatments, soil drenches, and foliar sprays. Due to widespread use, resistance developed rapidly and future formulations included multiple modes of action to extend the life of the product. The risk of resistance development is high, and products must be used as part of a resistance management plan. Once resistance has developed in an oomycete population, the pathogen is cross-resistant to all chemicals in this class.
FRAC group 7: SDHI: succinate dehydrogenase inhibition; carboxamides
Active ingredients and common/trade names: fluxapyroxad (Priaxor when blended with pyraclostrobin); fluopyram (ProPulse, blended with pyraclostrobin); boscalid (Endura); penthiopyrad (Vertisan); sedaxane (Vibrance)
Residual on plant tissue: Depends on the specific product; 10-30 days
Risk of fungicide resistance development: High
Mobility in plant: Not systemic
Protection of plant tissues: Protective
Mode of action: Succinate dehydrogenase complex II in the mitochondrial electron transport chain; Respiration
Carboxamides were first introduced in the 60s. Early products had a limited disease spectrum. Second generation products released since 2003 have an increased disease spectrum and potency. Due to the high risk of resistance development, these products are typically formulated as blends with other modes of action. Cross- resistance does occur but the patterns are complex. These products should be applied preventatively or early in disease development.
FRAC group 11: QoI (quinone outside) inhibitors a.k.a. strobilurin fungicides
Active ingredients and common/trade names: pyraclostrobin (Stamina, Headline), azoxystrobin (Quadris), picoxystrobin (Aproach), fluoxastrobin (Evito), also available in many blends with other MOAs
Residual on plant tissue: Depends on the specific product; 10-30 days
Risk of fungicide resistance development: High
Mobility in plant: Translaminar and systemic
Protection of plant tissues: Protective only
Mode of action: interferes with respiration, spore germination, penetration, and mycelial growth
QoI (strobilurin) fungicides are widely used for disease prevention. They were originally isolated from the wood- rotting fungus Strobilurus tenacellus and kill germinating spores. They are classified as a ‘reduced risk’ pesticide, indicating they pose less risk to human health than other chemical options at the time of registration by EPA. They have excellent preventative activity against a wide array of fungal diseases. QoIs have a high affinity for the leaf cuticle (waxy layer) and limited systemic activity, which means they can move within a leaf to which they’ve been applied. Mobility varies by individual active ingredient. Azoxystrobin can move systemically through the plant’s vascular system or ‘plumbing.’ Trifloxystrobin can move as a gas in the still layer of air (boundary layer) adjacent to the leaf surface. Several days may be required for full protection of the leaf via translaminar movement. QoIs have been known to show phytotoxicity, so check the label for restrictions on a crop. Phytotoxicity (toxic effects on plant tissues) may be increased by the use of crop oils, surfactants, and certain insecticides that solubilize the cuticle.
QoI fungicides have a high risk of fungicide resistance development because they have a very specific mode of action. Products in this class block electron transfer at the site of quinol oxidation (the Qo site) in the cytochrome bc1 complex, preventing ATP formation (energy) in mitochondria. A single point mutation in the gene responsible provides complete resistance. Resistance may be complete (qualitative) or may require increases in dosage over time (quantitative). In the example of QoI resistance in Ascochyta rabiei of chickpea, complete resistance is conferred, the mutation is retained in the population, and resistance to one QoI confers resistance to all QoI compounds (cross-resistance).
FRAC group M: Multiple sites of action and not classified
Specific MOAs, active ingredients and common/ trade names: inorganic M1: copper; inorganic M2: sulphur; dithiocarbamates M3: tetramethylthiuram disulfide (Thiram), mancozeb, maneb; pthalimides M4: captan, chloronitriles M5: chlorothalonil (Bravo); phenylpyridin-amine M29: fluazinam (Omega); not classified: oils, bicarbonates
Residual on plant tissue: Low
Risk of fungicide resistance development: Low
Mobility in plant: None
Protection of plant tissues: Contact for most. Fluazinam is locally systemic.
Mode of action: Various, depending on the product
FRAC group M fungicides have multiple sites of action, and are therefore at low risk of resistance development. The products act in various ways and some are approved in organic production. All have low residual activity and will have to be re-applied as the plant grows if the environment is favorable for disease.
- Copper products have efficacy against fungi and bacteria. Copper sulfate plus lime is one of the oldest fungicides used, and is known as ‘Bordeaux mixture.’ A safener such as lime reduces the phytotoxicity of copper. Bordeaux mixture can persist through rain, but needs to be re-applied to new tissues as the plant grows. The product is more phytotoxic at high temperatures (more than 85°F) and if rain occurs very soon after application. Copper is especially phytotoxic on younger tissues.
- Sulfur is the oldest known fungicide and has been used for over 2,000 years. Sulfur can be used as a preventative product against powdery mildew, rusts, and other diseases. Sulfur prevents fungal spore germination. Do not use sulfur when temperatures exceed 80°F or if you have applied an oil spray within the last month since the combination can be phytotoxic and cause crop injury.
- Dithiocarbamates, pthalamides and chloronitrilies were a major improvement in fungicide chemistry in the 40s to 60s due to their broad spectrum, low use rates, good efficacy, and low toxicity to mammals, plants, and the environment relative to organomercurial compounds and others. The dithiocarbamates remain the most widely used group of organic fungicides. The spectrum of efficacy varies by specific product.
- Phenylpyridin-amine is a broad-spectrum fungicide, and is locally systemic. It uncouples oxidative phosphorylation thus reducing fungal sporulation.
- Oils are mostly used for insect control and to prevent movement of viruses vectored by piercing-sucking insects such as aphids and thrips. They can help control these vectors and the viruses they spread. Oils are used in the management of powdery mildew, but phytotoxicity should be checked before widespread application. Do not apply when prevailing temperatures are less than 40°F or more than 90°F. It is best if relative humidity is less than 65% so the oil can evaporate quickly and reduce phytotoxicity.
- Bicarbonates can be effective when used in combination with oil. Ammonium and potassium bicarbonates are preferred to sodium bicarbonate due to buildup of sodium in the soil, which is phytotoxic. Some bicarbonates can add nutrients (nitrogen, potassium) to the soil.
Biofungicides
Biofungicides include biocontrol agents, in which one organism (bacterial or fungal) is used to control a plant pathogen directly, as well as products that induce plant defense responses for an indirect, induced plant resistance to the disease. Below is a list of currently available products on the market and their mechanism of action. Registered crops vary by product. A full list of products can be found in the label databases described above and the IR-4 Project Biopesticide database (http://ir4.rutgers.edu/biopesticides.html).
Bacillus subtilis strain QST 713 (Serenade ASO) induces the plant defense response. It is active on powdery mildew, downy mildew, Phytophthora, Alternaria, and Botrytis.
Bacillis subtilis strain GB 03 (Kodiak) competitive inhibition of growth of Fusarium, Rhizoctonia, Alternaria, and Aspergillis.
Bacillus pumilus strain GB34 (Yield Shield) for the suppression of Fusarium and Rhizoctonia.
Bacillus mycoides isolate J (LifeGard) induces the plant defense response. For use against Sclerotinia spp., early, late, and Cercospora blights.
Coniothyrium minitans strain CON/M/91-08 (Contans) is pathogenic on Sclerotinia. It colonizes and kills sclerotia of Sclerotinia spp. that cause white mold.
Fungicide resistance
Fungicide resistance is defined as ‘an acquired, heritable reduction in sensitivity of a fungus to a specific anti- fungal agent (or fungicide)’ (www.frac.info). Fungicide resistance occurs when a pathogen population that was previously sensitive to a fungicide is no longer controlled by the same fungicide. This is anecdotally known as a ‘field failure’ of the fungicide. Fungicides can lack activity on a fungal disease due to many factors including innate lack of efficacy, or using a fungicide at the wrong time, rate, or application method. Fungicide resistance develops over time with repeated exposure. The rate of resistance development is dependent on the pathogen itself, its life cycle, and the chemistry applied.
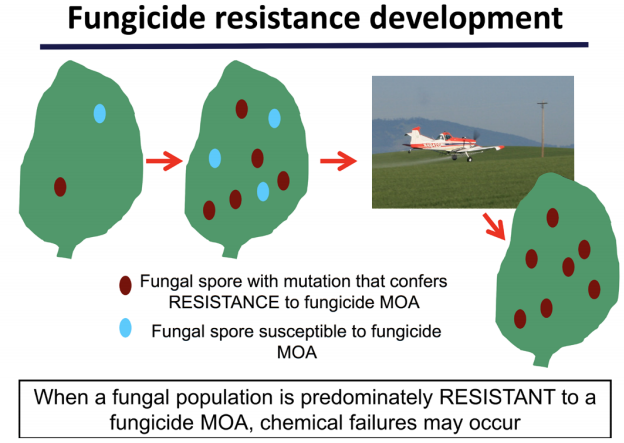
Fungicide resistance due to the pathogen: As with any pesticide, there is a risk that the pathogen targeted is either inherently resistant to the pesticide, or resistance to the pesticide will develop over time. Examples of inherent resistance exist because the mode of action is not effective on the targeted fungal pathogen. For example, DMI fungicides do not have activity on oomycetes. Fungicides are not generally effective on diseases caused by bacteria.
The influence of fungal life cycle on resistance development: Fungal pathogens can either have one life cycle per year or multiple life cycles per year. Pathogens with one life cycle per year infect plants, but do not spread to other plants from the initial site of infection. These are called monocyclic diseases. Diseases are monocyclic if there is one main infection period for the pathogen OR if there is only one time during the growing season that the plant is susceptible to the pathogen. Examples of fungal monocyclic diseases include soilborne root rots, crown rots, white mold, Fusarium head blight, and vascular pathogens such as Fusarium wilt. Monocyclic diseases must be managed with fungicide at the proper timing, which is generally once in the year. The risk of the pathogen population becoming resistant to a chemistry is considered lower than if the pathogen was exposed multiple times in a single year.
Pathogens that can infect a plant, then spread to new plants within the same growing season are called polycyclic diseases. These pathogens produce a reproductive structure on infected tissue, which then releases spores that can infect new plants. Fungal diseases are polycyclic if there are multiple spore releases OR the plant is susceptible to the pathogen over a long period of time. Examples of polycyclic fungal diseases include rusts and foliar blights. Polycyclic diseases may require multiple fungicide applications during a growing season, and therefore may be at higher risk of developing fungicide resistance.
Pathogen life cycles affect the exposure of the pathogen to the chemistry. For example, with concern about a soilborne fungus, apply a seed treatment. This is a single exposure per year, and the risk of the pathogen population becoming resistant to that chemistry is considered lower than if the pathogen was exposed multiple times in a single year. If the disease is foliar or on the reproductive structures including flowers and fruit, proper timing is important to target those tissues with foliar applications.
Fungicide resistance due to mode of action: As discussed above, different fungicide modes of action have varying risk of resistance development. The FRAC centralizes information on fungicide resistance worldwide and provides a forum for sharing information, including research. Please see their website for information on the mechanisms of fungicide resistance. In brief, fungicides with multiple sites of action have a low risk of resistance development. Many of the commonly used fungicides in field crops, such as the QoI, DMI, and SDHI groups, have a medium-to-high risk of resistance development. Cross-resistance to other fungicides within the mode of action is possible, and is common within the QoI group. The mutation responsible for resistance to QoI fungicides does not convey a fitness penalty, or reduced growth of the fungal pathogen, and is retained in fungal populations for long periods of time.
Reducing the risk of fungicide resistance development
When using fungicides for disease management, the principles of integrated pest management should be used to avoid resistance development. These include: Preventative cultural practices: Use best management practices including using high quality, pathogen-free seed, crop rotation, using an adapted crop variety, optimal seeding rate, planting date, irrigation practices, fertilization, sanitation including breaking the ‘green bridge,’ etc.
Monitoring: Scout crops for pests regularly and get them accurately identified. Use degree-day models where available to determine when the pest is likely to reach medium-high or high risk.
Acceptable pest levels: Determine what level of the pest will be tolerated.
Mechanical controls: Remove infected plants from the system to prevent reproduction and spread of the pathogen (rogueing).
Biological controls: Natural biological systems can mitigate pest damage. For plant diseases these can include beneficial insects that predate on or parasitize insect vectors of plant viruses and biological controls.
Responsible use: When a pesticide is needed, follow all label restrictions and use the best application methods possible to target the disease of interest. If lack of efficacy is suspected, leave untreated strip to compare with treated areas. If level of disease is the same, then a symptomatic sample should be sent to the diagnostic clinic.
Other recommendations to prevent fungicide resistance include:
- Select and use fungicides correctly
- Rotate the use of fungicide modes of action
- Limit number of applications of fungicides in a particular MOA each season: includes seed treatment
- Mix modes of action in blends or tank mixes
- Use fungicides at recommended rates
- Follow all label directions
3. Do the economics of the system justify the application?
Fungicides are available for use in field crops, fruit crops, turf, etc. In field crops, the economic value of a fungicide application needs to take into consideration the value of the crop, the price of the application, and the expected yield benefit of the application. The crop value and price of application are relatively easy to estimate. However, the expected yield benefit of the application is more difficult to estimate. This estimate factors in the whole disease triangle: the presence of disease (pathogen), the upcoming weather conditions (environment), and how the crop variety reacts to the disease (host). It must also factor in the efficacy of the product chosen, and efficacy given the application method and timing of application.
Pathogen: As discussed, get plants diagnosed and a disease confirmed if not familiar with the disease. Use Extension publications, websites, and the county Extension office and diagnostic lab as needed. Identification of the disease is extremely important prior to any pesticide application decision. Check the fungicide label for whether the fungicide is registered for management of the disease of concern. If the disease of interest is not on the label, it may not be effective and/or there may be phytotoxicity concerns for the product on the crop.
Environment: Every fungal disease has ideal environmental conditions for growth and infection. For example, stripe rust of wheat prefers relatively cool, wet weather and leaf rust of wheat prefers warmer temperatures associated with moisture. Ascochyta blight (develops on pulse crops) pathogen spores can be carried by wind to other areas, but this pathogen is primarily dispersed by rain splash on leaves. Knowledge about the environmental conditions that favor epidemics can help to make decisions based on upcoming weather or irrigation schedule which may favor disease development. In some cases, we can modify that environment. A common recommendation for Fusarium head blight of cereals is cutting irrigation around the flowering period to reduce moisture that favors disease until the crop is past the growth stage (flowering), during which the pathogen infects the head.
Host: Knowledge of a crop and variety tolerance to the pathogen of interest can be crucial when making spray decisions. Resistant or moderately resistant varieties reduce pathogen reproduction and spread to the point where fungicide is not necessary to limit disease spread. A susceptible variety may require multiple fungicide applications, depending on the disease of concern. Tolerant varieties have visible disease symptoms but do not lose significant yield or quality when affected by the disease.
Another aspect that must be considered is the plant age when the disease is a threat. First, some diseases such as white mold or fusarium head blight have a specific stage (flowering) during which they infect the plant. When the crop is not at this stage, it is not susceptible to the pathogen. Second, crop age can influence susceptibility. In general, the later the infection occurs, the less loss will be observed. Thirdly, age-related resistance can be a factor. Some varieties are susceptible during early vegetative stages, but as adults are not susceptible. An example is adult plant resistance for stripe rust of wheat. Lastly, some crops are not susceptible to the disease of concern. This is a primary reason crop rotation is used for disease management.
Product efficacy: Not all fungicides are effective against all diseases. Check the label to make sure the crop and the targeted disease are listed. Efficacy ratings for fungicides can generally be found from university extension and researchers, but not always. A crop consultant and chemical sales representative can be helpful, but always check the labels and know what is being applied. Follow the directions on the pesticide label for best efficacy.
Product application: The specific product, rate used, timing of application, amount of water, nozzle type and equipment used for application can all affect efficacy on the disease. Check the label to determine recommendations and restrictions. Water pH, surfactants, blending with other products, etc. can affect product efficacy and phytotoxicity.
The level or severity of the disease.: Some diseases can occur at low levels without causing significant yield loss, e.g. brown spot in soybeans and tan spot in wheat.
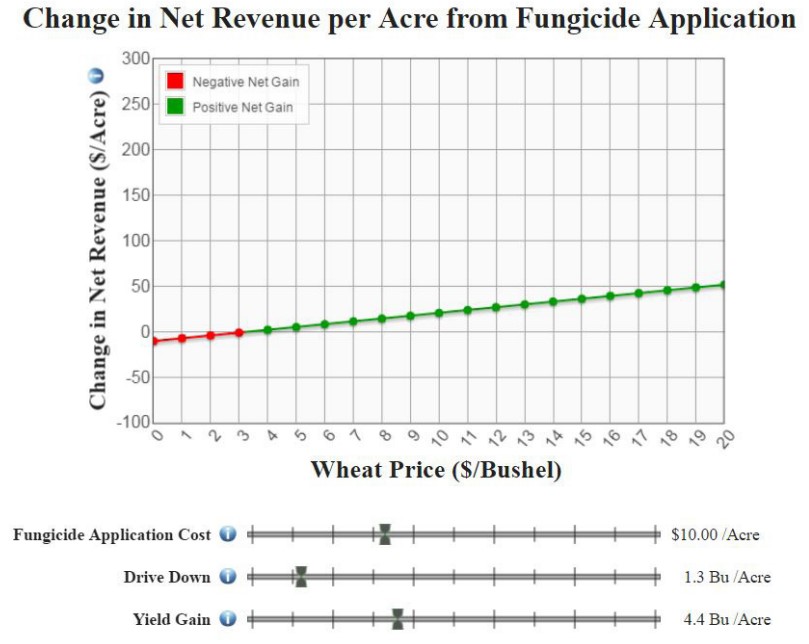
The MSU Extension Fungicide Decision Tool can help inform the choice of whether to apply fungicide by calculating the change in net revenue from fungicide application, over a range of wheat prices. An example using the decision tool is shown below.
By adjusting the slider bars, change the fungicide application cost, drive-down (loss from wheel tracks when spraying, if applicable), and expected yield gain from the application. The line on the graph is red for a negative change and green for a positive change. In the example here, the grower’s fungicide application cost is $10 per acre. Note that this cost includes not only the cost of the fungicide, but also the cost to operate the sprayer or other equipment used in application. The expected drive-down from application is 1.5 bushels per acre; some guidelines for estimating drive-down are provided on the website. If the fungicide is applied along with an herbicide or other chemical, the cost of application and drive-down may be less for each chemical as the equipment costs can be split between them. The yield gain from fungicide application is estimated at 4.2 bushels per acre. Note that there are wide ranges in estimates of the yield benefit from applying fungicide. For this operation, the benefit from fungicide application exceeds the cost when the price of wheat is $4 per bushel or more.
Glossary
Acropetal pentrant: Fungicide moves into the plant tissue then upward through the xylem, or water conducting tissue, of the plant
Contact fungicide: Must come into contact with the fungus to have anti-fungal activity; is not absorbed by plant tissues and does not move beyond the site of application.
Cross-resistance: Acquisition of resistance to one chemical in the FRAC group confers resistance to closely related fungicides without exposure of the pathogen to that class of fungicide.
Curative: A curative fungicide stops early growth of the fungal pathogen (colonization of plant tissues). They act against hyphal elongation. Most prevent early infection and have protective activity. They are most effective when applied before infection or in the first 72 hours after infection.
Dose: The amount of product, or concentration of the product.
Fungicide Resistance Action Committee: An organization that classifies fungicides by mode of action and tracks fungicide resistance worldwide
Fungistatic: Most fungicides are only fungistatic. They prevent growth or development of fungi and do not actually kill them.
Hyphae/hyphal: The growing portion of a fungus, like a root of a plant.
Localized penetrant: Fungicide that is absorbed in plant tissues, but does not move significantly beyond the site of uptake.
Mode of Action: The biochemical mechanism by which a pesticide has activity against a pest of interest.
Monocyclic: The disease-causing pathogen has one infection cycle per growing season. Typical of soilborne plant pathogens.
Multi-site mode of action: Interferes with two or more biochemical processes in susceptible fungi.
Mycelia: vegetative growth of fungi consisting of network of hyphal growth
Parasitize: To infest or live in or on a host.
Penetrant: Moves into plant tissues and can move in xylem of the plant. Does not move downward into the roots.
Phytotoxicity: Damage or other harm caused by the application of a pesticide on a plant. Examples include yellowing (chlorosis), leaf burning, stunting, etc.
Polycyclic: The disease-causing pathogen has more than one infection cycle per growing season. Typical of foliar fungal pathogens.
Predate: To act as a predator of.
Protective: Protective fungicides must be applied before the fungus contacts the plant tissue. They act very early in the infection cycle, during spore germination.
Qualitative fungicide resistance: A type of fungicide resistance during which the pathogen population exhibits significantly higher resistance to the fungicide. The larger doses required to control resistant isolates may be impractical at a field scale.
Quantitative fungicide resistance: A type of fungicide resistance where the dose of fungicide required to reduce fungal populations increases in a step-wise manner over time.
Residual: Fungicides are active for a period of time after application, this is often known as the ‘residual period.’
Resistant: Fungal pathogen growth is not prevented by the chemical. This can be due to innate resistance or acquired resistance to the fungicide applied.
Rogueing: Removing infected plants mechanically (pulling, tilling, etc.) so they cannot serve as a source of disease for healthy plants.
Spore: a unit of sexual or asexual reproduction. Used for survival and dispersal, often over extended periods.
Sterol: A group of naturally occurring steroid alcohols which occur in plants, animals and fungi. Commonly they occur in cell membranes and are a site of activity for DMI fungicides.
Susceptible: Fungal pathogen growth is prevented by the chemical.
Systemic: No fungicides registered on field crops are truly systemic. None move throughout plant from leaves to roots and vice versa. Most fungicides referred to as systemic are more accurately penetrants.
Tolerance: In the case of plant yield, varieties which can become infected with a pathogen yet not lose significant yield are called ‘tolerant.
Xylem mobile: Fungicide moves in the xylem tissues of the plant, which conduct water from roots upward
For more information
Fungicide Resistance Action Committee website: www.frac.info
Klittich, C. J. 2008. Milestones in fungicide discovery: Chemistry that changed agriculture. Online. Plant Health Progress doi:10.1094/PHP-2008-0418-01-RV.
Morton, V. and Staub, T. 2008 A Short History of Fungicides. Online, APSnet Features. doi: 10.1094/ APSnetFeature-2008-0308.
Mueller, D., Wise, K., Dufault, N., Bradley, C. & Chilvers, M. (eds.) 2013. Fungicides for Field Crops, St Paul, MN: APS Press.
Pscheidt, J. Fungicide Theory of Use and Mode of Action. Pacific Northwest Handbook. https://pnwhandbooks.org/plantdisease/pesticide-articles/ fungicide-theory-use-mode-action
Common chemical and trade names are used in this publication for clarity of the reader. Inclusion of a common chemical or trade name does not imply endorsement of that particular product or brand of herbicide and exclusion does not imply non-approval.
Pesticide usage suggestions provided in MSU Extension materials are intended to serve only as a guide and are published for educational purposes. If any suggestions conflict with a product label, follow the product label instructions. Read and follow all product labels carefully.

