
Western Salsify
Western salsify, also known as goatsbeard, is an exotic plant that can form dense stands that displace other vegetation and decrease forage production. This publication will describe how to identify western salsify, explain its biology and ecology, and discuss management including the results of a research trial conducted in north-central Montana.
Last Updated: 10/17by Jane Mangold, MSU Extension Invasive Plant Specialist, and Allison Lansverk, Research Assistant
WESTERN SALSIFY (TRAGOPOGON DUBIUS), ALSO
known as goatsbeard or yellow salsify, is an exotic plant of the Asteraceae family that can be weedy in rangelands, pastures, Conservation Reserve Program (CRP) lands, and roadsides throughout North America. The name “salsify” means “a plant that follows the sun,” aptly named because open flowers point towards the sun and follow it across the sky, often closing by late morning or early afternoon. The term “goatsbeard” comes from a reference to the conspicuous pappus on the flowerheads gone to seed as observed by the third century B.C. Greek philosopher Theophrastus, indicating this plant has been associated with people for a long time. (Terms in bold can be found in the glossary on page 4.)
Native to Eurasia and northern Africa, western salsify was commonly used as a food plant in northern Europe in the Middle Ages and subsequently spread all around the world. It was brought to North America by early settlers as a food plant and ornamental around the turn of the 20th century. Currently, western salsify is widespread across North America. It has been reported in every state except Alabama, Florida, Mississippi, and South Carolina; in every Canadian province except Newfoundland; and in all counties in Montana. Western salsify can be problematic in rangeland and CRP in north-central Montana where it has been observed to form dense stands that replace other vegetation and decrease forage production.
Identification
Western salsify has linear, grass-like leaves that clasp the stem at the base. Prior to bolting and flowering, the leaves can be readily mistaken for grass (Figure 1). However, they have a smoother and more rubbery-like feel than grass leaves, have hairs in the axils, and exude a milky juice when broken. The yellow, dandelion-like flower heads are born singly on 12 to 40 inch (30 to 100 cm) stems that are swollen and hollow below the flower head (Figure 2). Ten or more long, narrow involucral bracts extend beyond the flowers. There are 20 to 120 ligulate flowers per head. The flowerhead matures to form a three to four inch diameter fluffy sphere comprised of seeds with long, slender beaks and a white, umbrella-like pappus that aids wind dispersal (Figure 3). The fluffy sphere of seeds looks similar to that of a dandelion, only much larger. Western salsify has a thick, fleshy taproot.

FIGURE 1. Western salsify rosette.

FIGURE 2. Western salsify flower.

FIGURE 3. Fluffy sphere of western salsify seeds.
Other Salsify Species in Montana
Several salsify species grow across North America, and four species in addition to western salsify have been reported in Montana. Those include meadow salsify (T. lamottei; T. pratensis), common salsify (T. porrifolius), Moscow salsify (T. miscellus), and remarkable goatsbeard (T. mirus). Leaves and milky juice of meadow salsify are similar to those of western salsify. The flower is also yellow, but the bracts do not extend beyond the flower head, and the flower stalk is not hollow or swollen beneath it. Common salsify is also similar in appearance to western salsify, but the flower is purple instead of yellow, and the seed pappus is brownish instead of white. Moscow salsify is a hybrid of western and meadow salsify, while remarkable goatsbeard is a hybrid of western and common salsify. The hybrids typically have characteristics intermediate between the two parents. Moscow salsify and remarkable goatsbeard allegedly escaped from the Washington State University garden in Pullman, Washington, prior to the 1950s. Meadow salsify has been reported from 22 Montana counties; common salsify has been reported from eight Montana counties. Moscow salsify has been reported in Flathead, Madison, and Carbon counties, whereas remarkable goatsbeard has been reported in Lake and Judith Basin counties.
Ecology and Biology
Western salsify is a monocarpic perennial dependent upon seed production to maintain and spread populations. Being monocarpic, the plant dies after seed production. This can happen in its first to fourteenth year (rarely), but usually after two to four years. Seed viability has been measured at 94 percent, and both primary and secondary dormancies have been exhibited by salsify. Secondary dormancy is induced by low oxygen conditions typical of deep burial (one to two inches) or inundation by water. Dry storage releases seeds from secondary dormancy.
Each flower head produces about 20 to 120 seeds of two types; a heavier, darker seed is produced from the outer ring of florets on the flower head, while a lighter seed is produced from the central florets. This difference in seed morphology may result in differences in germination potential and dispersal characteristics. For example, research suggests that smaller seeds from the center florets result in smaller seedlings. However, when growing in competing vegetation, seedling size decreased as seed size increased, whereas when growing in competing vegetation plus plant litter, seedling size increased as seed size increased. Additionally, the dark peripheral seeds, which tend to be larger, have a terminal velocity that is 1.3 times greater than the central seeds, which suggests lower dispersal potential.
Germination peaks have been observed in the fall and the following spring. Over a thirteen month period, all but three percent of a seed crop germinated indicating little contribution to the long-term seed bank. Light is not required for germination, and emergence is not inhibited by a canopy of vegetation or litter. Seedlings quickly gain access to light by the growth of long narrow cotyledons through competing vegetation or litter. Rosettes have an erect growth form (Figure 1) which allows them to colonize intact plant communities. An extensive root system is developed during the vegetative life history stage. Carbohydrates are stored in both the thick roots and in the rosette leaves. The initiation of flowering may be induced by a cold period. When rosettes develop over a prolonged vegetative phase with little flowering, the increase proportionally of rosettes in the population develops a “bud bank” that compensates for the lack of representation in the seed bank.
Flowering occurs in early to mid-June and can extend into September. Only one flower head is produced per stalk, but plants may produce several flowering stems, ranging from one to 14 flowers per plant. Flowers are insect pollinated. One study found an average of 90 seeds produced per plant. Wind- dispersed seeds may travel up to 825 feet (250 m).
Western salsify will grow across a variety of soil types ranging from sandy to clay loam. While it is frequently found in highly disturbed sites, it also occurs in less disturbed areas as well and grows in a wide variety of vegetation zones ranging from arid grasslands to mesic forests. Like other weeds, it can form dense stands that displace other vegetation and decrease forage production and plant diversity. For example, research in British Columbia, Canada, suggested that western salsify reduced the leaf area and shoot-to-root ratio of bluebunch wheatgrass, an important component of native rangeland.
Various parts of western salsify are consumed by wildlife. In Oregon, western salsify was shown to be one of the most important plant foods of blue grouse (Dendragapus obscurus) during the fall. Western salsify, along with four other species, made up 68 percent of the blue grouse diet by weight. Flowering stalks and foliage are utilized by a variety of mammals. For example, pocket gophers (Geomys bursarius) frequently feed on salsify roots. Other mammals such as deer, squirrels, or rabbits may bite off one or more flowering stalks. Mature plants tend to be grazed less frequently.
Management
Very little information exists regarding western salsify control. Research in Canada estimated four years of control using picloram at one pint per acre and one year control using dicamba at two quarts per acre. Herbicides currently labeled for western salsify control include Chaparral® (metsulfuron methyl + aminopyralid) at 3.0 to 3.3 oz/A (ounces per acre), Cimarron® Plus or X-tra (chlorsulfuron + metsulfuron methyl) at 1.25 to 2 oz/A, and Escort® (metsulfuron methyl) at 1 to 2 oz/A.
While not tremendously problematic, small infestations of western salsify can be hand-pulled or dug. Research suggests burying seeds greater than three inches deep prevents emergence, so tilling may be effective, but not recommended unless tilling is followed by seeding of desirable plants. Western salsify can be a seed contaminant, so buying weed-free, high quality seed can help prevent introductions on cropland, pasture and conservation seedings.
To help develop herbicide management of western salsify, research trials were conducted in north-central Montana on CRP lands with varying degrees of infestation. In spring and early summer 2010, a variety of treatments (Table 1) were tested at three sites of varying infestation density [low (~1 flowering plant/m2), medium (~3 plants/m2), and high (~34 plants/m2)].
All herbicide treatments were applied with 0.10 percent nonionic surfactant (Penetrator®) plus 0.10 percent water treatment solution (BroncMax®). Glyphosate was a 4-lb formulation and 2,4-D was an LV-6 formulation. Herbicide treatments were applied with a CO2 backpack sprayer delivering about 16 gallons of water per acre at 40 to 42 psi. Mowing was accomplished once at the bolting/flowering stage using a standard push mower set to mow to a four to six inch stubble height. Density and biomass were sampled in early August for two years (2010 and 2011).
| Treatment | Timing |
| 1. glyphosate (4) +2,4-D (3) | rosette |
| 2. dicamba (4) + 2,4-D (10) | rosette |
| 3. dicamba (2) + 2,4-D (10) + metsulfuron (1/10) | rosette |
| 4. dicamba (4) + 2,4-D (10) | bolting/flowering |
| 5. dicamba (2) + 2,4-D (10) + metsulfuron (1/10) | bolting/flowering |
| 6. glyphosate (4) +2,4-D (3); | rosette; |
| dicamba (2) + 2,4-D (10) + metsulfuron (1/10) | bolting/flowering |
| 7. mowing | bolting/flowering |
| 8. no management (control) |
TABLE 1. Management treatments and their timing of application (rosette = 15 May and bolting/flowering = 20 June) to low, medium and high density salsify infestations in north-central Montana. Treatment 6 had two application timings: glyphosate plus 2,4-D at rosette stage and dicamba plus 2,4-D, plus metsulfuron at bolting/flowering. Numbers in parenthesis following the chemical name are the chemical rates in ounces product per acre.
When compared to the no management control, treatments significantly reduced western salsify flowering and rosette plants only at the high density site. This suggests there may be a threshold salsify population density below 34 plants per square meter, below which there is no benefit to management. Conversely, medium densities of salsify (~three plants per square meter) may be beneficial for wildlife habitat, particularly for blue grouse and the closely related sage grouse.
At the high density site in 2010, flowering salsify plants were only reduced compared to the no treatment control by treatments 2 and 6 (Figure 4A). Timing of application appeared to be important, in that treatment during the rosette stage generally outperformed later applications during bolting/flowering (e.g. treatment 2 versus treatment 4). However, by 2011 all herbicide treatments reduced flowering plant density compared to both the mowing treatment and the control (Figure 4A). When compared to the control, all herbicide treatments reduced rosette densities in 2010 (Figures 4B). These results support the idea that herbicides that target the rosettes, or the bud bank, will effectively reduce salsify populations in the short term. In addition, all herbicide treatments reduced the salsify population to a level that may still provide benefit to wildlife.
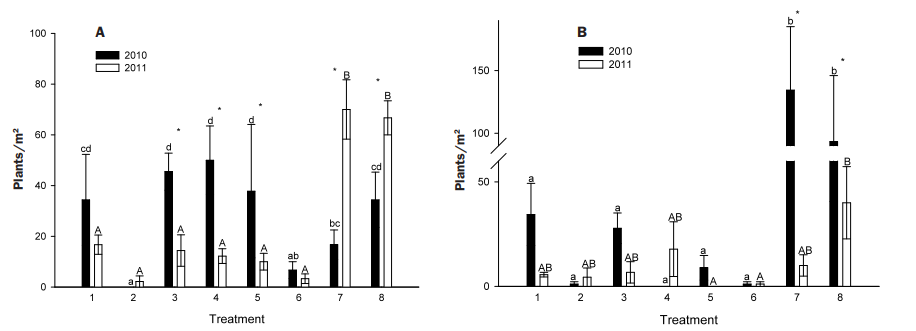
FIGURE 4. Treatment effect on western salsify flowering plant density (A, left) and rosette density (B, right) across years at a site in north-central Montana with a high degree of infestation. Treatments are described in Table 1. Lower case letters separate means across treatments within 2010, while upper case letters separate means across treatments within 2011. ‘*’ indicates where means differed between 2010 and 2011 within a treatment.
Mowing reduced neither the flowering nor rosette salsify density compared to the control. From 2010 to 2011 there was a similar flowering plant density increase in the mowing and control plots (Figure 4A), and a similar decrease in rosette densities (Figure 4B). The increase in light from canopy removal through mowing may have resulted in more robust rosettes that flowered in 2011. This is supported by the large increase in the number of flowering plants from 2010 to 2011 in the mowing plots. Therefore, results do not support mowing as a management recommendation to reduce salsify, which suggests grazing management may not be effective either.
Perennial grass density and biomass were highest in treatment 2. Weedy annual grass densities only increased in treatments 5 and 6. All other treatments had annual grass densities similar to the control.
The results support a recommendation of four ounces of dicamba combined with 10 ounces of 2,4-D applied at the rosette stage (treatment 2, Table 1) for managing salsify because it provided effective control of western salsify and resulted in an increase of perennial grasses without stimulating annual grasses. This study supports management of high density sites since western salsify flowering plants increased from 2010 to 2011 in the mowed and non-treated sites, suggesting continued increase and spread if left untreated. Treatment differences were not detected at the medium and low density sites probably because of the patchy distribution of plants. Broadcast herbicide application to treat western salsify in such situations may not be necessary, but spot treatment of dense patches may prevent infestations from worsening.
Glossary
Beak - A long slender tip or projection
Bolting - When a plant extends a flowering stem
Cotyledon - Embryonic leaf in the seed, often persisting in the seedling as lowermost leaves or leaf-like structures (two in dicots, one in monocots)
Involucral bracts - A whorl or series of closely arranged bracts (reduced leaves) below a flower
Monocarpic perennial - Reproducing in a single bout during a lifetime, usually dying afterwards
Pappus - Modified calyx (outermost series of flower parts; sepals) in flowers of the Asteraceae family, consisting of bristles, scales, awns, or a short crown at tip of achene
Rosette - Cluster of leaves radiating out in all directions from the stem, usually at base of plant
Common Salsify as an Edible Plant
Although the roots of all the salsify species found in North America are edible, common salsify is the species that is widely cultivated as a vegetable. This plant is often called “vegetable oyster” or “oyster plant,” referring to the faint oyster-like flavor possessed by the roots. The parsnip-like roots can be harvested in spring, but the flavor is reportedly enhanced if roots are harvested after a freeze in the fall. A botanist from the 16th century described the taste as surpassing that of carrots or parsnips. The greens may also be eaten and have a sweet taste. Uses for common salsify range from soup and salads to a dietetic medicine. The use of common salsify as a vegetable plant has primarily occurred in Europe and has declined over time.
References
Clements, D.R., M.K. Upadhyaya, and S.J. Bos. 1999. The biology of Canadian weeds. 110. Tragopogon dubius Scop., Tragopogon pratensis L., and Tragopogon porrifolius L. Canadian Journal of Plant Science 79:153-163.
Mangold, J.M. and A.L. Lansverk. 2013. Testing control options for western salsify (Tragopogon dubius) on Conservation Reserve Program Lands. Weed Technology 27:509-514.
Acknowledgements
The authors would like to thank USDA-Natural Resources Conservation Service and USDA-Farm Services Agency for financial support; John Good, Logan Good, and Randy Vischer for cooperating on the research trials; and Jim Jacobs, Jesse Fulbright, and Bill Evans for reviewing an earlier version of this document.

