
Small Grain Root and Crown Diseases
Proper diagnosis of root and crown diseases will help producers make informed management decisions. In this publication we discuss the symptoms and control of the various root and crown diseases common to cereal crops in Montana.
Last Updated: 07/13by Mary Burrows, Assistant Professor and Extension Plant Pathology Specialist; Alan Dyer, Assistant Professor, Plant Sciences and Plant Pathology Department; and William Grey, Director, Montana Foundation Seed Program
ROOT AND CROWN ROTS OFTEN GO UNNOTICED,
reducing yield until large patches of fields are missing plants or white, empty heads appear at crop maturity. Best management practices occur at planting and include using a seed treatment, crop rotation and variety selection. There are no curative treatments for root and crown diseases. Positive identification and specific management recommendations for root and crown diseases can be obtained from your local county Extension office.

FIGURE 1. Dry seed decay occurs when there is insufficient soil moisture for germination and seedling establishment. Note the green fungus growing on seed. (Jeff Johnston)
Damping off
Poor emergence is often the first sign of damping off. Damping off can occur before plant emergence or in the young seedling (post-emergence damping off). Symptoms include poor stand, young, yellow seedlings with poor root systems, and plant death.
Damping off is caused by a number of pathogens including Pythium spp., Fusarium spp., Rhizoctonia spp., and Cochliobolus spp., among others. It is soil borne and has increased with no-till management due to cooler, wetter soils in the spring. Damping off organisms such as Fusarium, Cochliobolus and Alternaria can also be carried on the seed.
Management
Plant high quality seed and always use a fungicide seed treatment. The selection of seed treatment will depend on what is available at your retailer. Avoid planting in cold, wet soils when possible. Tillage can bury plant residue which contributes to damping off at the soil surface and increase the soil temperature which facilitates rapid seedling emergence.
Dry Seed Decay
Dry seed decay occurs when seed is planted without sufficient moisture for germination and seedling establishment (Figure 1). It is caused by Penicillium, Aspergillus and other fungi which are present in the soil. Most fungicide seed treatments are effective at preventing dry seed decay for two to three weeks following planting.
Root and Crown Rots
Early season root rots
Symptoms appear after planting or in the seedling stage. Cool wet weather after seedling emergence often results in a seedling root rot. Symptoms generally occur in circular patches (Figure 2, page 2) and will include stunted, yellow plants and may be mistaken for nitrogen deficiency. When the plant is dug up the roots will be much thinner than those on a healthy plant or there may be no secondary roots at all. Roots will be discolored, and the color and pattern of discoloration depends on the pathogen infecting the roots (see below). The three main types seen in Montana are Pythium root rot, Rhizoctonia root rot (bare patch), and common root rot. Fusarium spp. can also cause root rot. These fungi infect a very broad host range, so crop rotation is of limited efficacy.

FIGURE 2. Characteristic circular pattern of root rot at the tillering stage - in this case, a combination of Pythium and Rhizoctonia. (Mary Burrows)

FIGURE 3. Characteristic ‘spear-tipping’ symptom caused by Rhizoctonia root rot. (Mary Burrows)
Pythium root rot is caused by the “water mold” (oomycete) pathogen, Pythium spp., that produces zoospores which move in soil water. The disease is characterized by poor root system development and a lack of fine root hairs which can be difficult to diagnose when the plants are pulled from the soil. In general, roots will be brown in color and the outer layer (root cortex) will peel off, leaving a thin strand of tissue (the root stele). Pythium is common in Montana soils and survives for years as a structure called an oospore. Crop rotation is not effective as the pathogen will infect most crops. Because many of the spring cereals are planted into cool soils in Montana, it is recommended that all seed treatments contain metalaxyl or mefanoxam for control of Pythium.
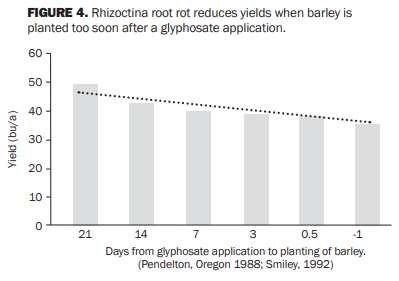
Rhizoctonia root rot (bare patch) is caused by the fungus Rhizoctonia solani. It is first diagnosed by poor stands of spring crops or declining stands of winter wheat in the spring. It is diagnosed by poor root development and ‘spear-tipping’ of the fine roots (Figure 3). Roots are short and narrow at the tip and dark brown or black (Figure 3). Rhizoctonia root rot is very common where volunteer grain and weeds were sprayed with herbicide, particularly glyphosate, within days of planting (Figure 4). The fungus grows rapidly by feeding on the flush of root nutrients from the dying plants and ‘bridges’ to the newly planted cereal crop. This is called a ‘green bridge.’ The green bridge can be prevented by delaying seeding two to three weeks after herbicide application to allow the damaged tissues to decompose (Figure 4).
Later season root rots
White heads or poor grain fill are often the first time a producer will notice the effects of crown and root rots. The fungus infects much earlier in the season, and symptoms generally appear at the crown and subcrown internode (between the seed and soil level) around flowering. The color and pattern of discoloration are distinctive for each of the crown rots, as illustrated in the following sections.
Common root rot is caused by the fungus Bipolaris sorokiniana (also referred to as Cochliobolus sativus). It can easily be confused with take-all and Fusarium crown rot, but is the most common crown rot in Montana. Above- ground symptoms are most recognizable at maturity when there are stunted plants, a reduced number of tillers, small heads, shriveled grain, premature ripening, and white heads. Crown symptoms consist of brown (but not shiny) streaks at the base of the plant, which rarely extend above the first node (Figure 5). There are often eye-shaped, dark brown to black lesions on the subcrown internode where the fungus is starting to invade.
Management recommendations include crop rotation to a non-cereal, use of a fungicide seed treatment, and promoting plant health by reduction of water, planting at the appropriate depth (approximately one inch), nutrient and herbicide injury stress.

FIGURE 5. Symptoms of common root rot on the lower stem of wheat. (Mary Burrows)

FIGURE 6. Symptoms of Fusarium crown rot on the lower stem. (Ernesto Moya)

FIGURE 7. Mild to severe take- all symptoms on the crown of infected plants. (Mary Burrows)
Fusarium root, crown, and foot rots are caused primarily by Fusarium culmorum, F. pseudograminearum, and F. graminearum. Disease is most severe following a wet spring and a summer of drought, fluctuating water conditions, and high soil nitrogen levels. Above-ground symptoms include a characteristic chocolate brown discoloration that can extend into the second node (Figure 6). Below- ground symptoms include a tan-brown discoloration of the subcrown internode. Plants mature early, produce shriveled or no seed, and white heads. A diagnostic pink, cottony mycelium can fill the stem or grow between the leaf sheath and stem under high humidity.
Management recommendations include fungicide seed treatments, agronomic practices to minimize water stress during grain fill, including planting at the appropriate depth, minimization of vegetative growth by avoiding high fertility, seeding winter wheat in late autumn to limit vegetative growth and prevent water stress on the plants, crop rotation to broadleaf crops, adequate but not excess soil fertility, and selecting varieties with adaptation to the prevalent growing conditions.
Variety recommendations for Fusarium crown rot can be found below for spring wheat (Table 1) and durum wheat (Table 2). The disease is most severe in spring crops, but winter wheat can also be infected. Winter wheat inoculated trials have not been consistent enough to provide specific variety recommendations.
TABLE 1. Performance of spring wheat varieties challenged with Fusarium crown rot (Dyer and Kephart, Huntley, 2005)
Variety |
Yield (bu/A) | Percent yield reduction |
|
| Control | Inoculated with Fusarium crown rot | ||
| Alsen | 60 | 53 | 12 |
| Choteau | 55 | 43 | 22 |
| Explorer | 49 | 44 | 10 |
| Hank | 58 | 48 | 18 |
| Knudsen | 60 | 47 | 21 |
| McNeal | 51 | 46 | 10 |
| NorPro | 57 | 45 | 21 |
| Outlook | 62 | 53 | 13 |
| Reeder | 58 | 45 | 22 |
| Vida | 64 | 50 | 22 |
| Mean | 57 | 47 | 17 |
Take-all (Gaeumannomyces graminis var. tritici) causes the greatest damage on winter and spring wheat that is grown under irrigation or high precipitation. Take-all is also a pathogen to a lesser extent on barley, brome and wheat grasses, quackgrass, rye, and triticale. Symptoms are most obvious near heading, when the crop appears uneven in height, and plants have premature blighted (white) heads. Diseased plants are easy to pull from the soil since the roots are short and few in number. Symptoms on the crown include an obsidian black, shiny discoloration (Figure 7).

FIGURE 8. Eyespot (Strawbreaker) lesion on a lower stem. (Mary Burrows)

FIGURE 9. Symptoms of Cephalosporium stripe on winter wheat. (Jeff Johnston)
Management is best achieved with crop rotation to non-cereal crops or continuous wheat planting when associated with 'take-all decline'. Destroy grassy weeds and volunteers which can harbor the pathogen. Late planting of winter wheat can help reduce the likelihood of infection. No-till wheat production can result in improved soil structure and porosity and can reduce the impact of take-all disease. A take-all decline phenomenon, which is a gradual reduction in disease severity, can occur when wheat is grown continuously for many years. The decline of disease is caused by the competitive soil microflora that directly parasitize the fungal pathogen or through production of anti-fungal compounds by soil borne bacteria, especially the root colonizing fluorescent Pseudomonas spp.
TABLE 2. Performance of durum wheat varieties challenged with Fusarium crown rot (Dyer and Kephart, Huntley, 2005)
Variety |
Yield (bu/A) | Percent yield reduction |
|
| Control | Inoculated with Fusarium crown rot | ||
| AC Avonlea | 53 | 40 | 25 |
| Ben | 49 | 37 | 24 |
| Kyle | 32 | 27 | 16 |
| Lebsock | 53 | 40 | 25 |
| Maier | 51 | 39 | 24 |
| Monroe | 55 | 46 | 16 |
| Mountrail | 47 | 38 | 19 |
| Vic | 47 | 37 | 21 |
| Ward | 53 | 40 | 25 |
| Mean | 49 | 38 | 25 |
Eyespot/Strawbreaker foot rot (Pseudocercosporella herpotrichoides) causes an eyespot lesion on the lower stem. The lower stem lesions are eye-shaped, brown in color, often have a dark center and cannot be removed by stripping off the leaf sheaths (Figure 8). The negative yield effect is due to lodging, delayed harvest and difficulty in picking up the heads.
Eyespot is favored by high moisture, a dense canopy, continuous cereal crops, early seeding, and high soil nitrogen levels. In hot, dry weather, infected stems stressed by moisture loss can die. Infection occurs in the early spring, and spring wheat escapes initial infection due to planting date. Lowering seeding density increases air flow in the canopy and may reduce disease. Other management recommendations include delayed seeding to avoid the initial inoculum and crop rotation to non- cereal crops for two years to allow host debris to decay.

FIGURE 10. Death of plants caused by snow mold. (Tim Murray, Washington State University)
Fungicides applied at the beginning of stem elongation (Feekes growth stage 6) have been used widely in the Palouse region of the Pacific Northwest. Strains of the fungus are resistant to benzimidazole fungicides (benomyl, carbendazim, thiabendazole, thiophanate-methyl). Currently, triazoles (cyproconazole, epoxiconazole, flusilazole, and propiconazole), prochloraz and cyprodinil are used for eyespot control. Spraying combinations of active ingredients may reduce the development of fungicide resistance in the pathogen. Plant resistance genes, Pch1, have been deployed in the club and bread wheats, where the disease has been most damaging.
Cephalosporium stripe (Cephalosporium gramineum) is a disease of winter wheat. Symptoms include longitudinal yellow stripes running the entire length of the leaf blade, culm, and leaf sheath, often accompanied by dark green veins. The stripes may turn necrotic, the internode below nodes is darkened, and the linear stripes will appear dark brown on a light chaff wheat variety as the plant nears harvest. It is favored by wet soil, fluctuating winter temperatures, hard pan soils, and continuous cropping of winter cereals and grasses.
Infection occurs primarily in the late winter as a result of soil heaving, which damages roots and provides an entry for fungal spores. The fungus causes a true vascular wilt by plugging the xylem element, or water conducting tissues of the wheat plant. Spring wheat escapes infection since it is planted after the infection period. Differential reaction by varieties does appear to occur as some are less damaged than others, however, there is no true resistance to Cephalosporium stripe (Table 3).
TABLE 3. Performance of winter wheat varieties challenged with Cephalosporium gramineum, the pathogen causing Cephalosporium stripe (Dyer, Bozeman, 2007).
Cultivar |
Yield (bu/A) | Percent yield reduction |
|
| Control | Inoculated with Cephalosporium stripe | ||
| Bynum | 90 | 83 | 8 |
| Falcon | 107 | 100 | 7 |
| Genou | 99 | 81 | 18 |
| Jagalene | 104 | 71 | 32 |
| Ledger | 101 | 91 | 10 |
| Norris | 100 | 90 | 10 |
| NuSky | 88 | 88 | 0 |
| Rampart | 97 | 93 | 4 |
| Yellowstone | 112 | 86 | 23 |
| Mean | 101 | 88 | 13 |
Snow molds (speckled, Typhula spp. and pink snow mold, Microdochium nivale) occur when winter wheat is covered by snow for extended periods and during cool, wet periods in the fall and spring. It can be a significant cause of winterkill. Symptoms include death of plants and lesions on leaves (Figure 9). In humid weather, fungal mycelia can be seen growing on the plants. Typhula produces dark ‘specks’ on leaf tissue which are actually fungal structures called sclerotia. This disease is more associated with prolonged snow cover than pink snow mold. M. nivale produces oblong lesions on leaves with straw-colored centers and dark margins. Under moist conditions, pink to peach fungal mycelium can be seen growing from plant tissue and stubble.
Early planting allows the plant to establish and favors recovery from the disease. Growing spring cereals effectively controls the disease. Fungicide seed treatments may offer limited protection in small grains, particularly pyraclostrobin (Stamina). Foliar fungicides will mitigate disease in turf grass, but are rarely effective in small grain production.
Sharp eyespot is caused by Rhizoctonia cerealis. It is common in continuous cereal crops where the fungal sclerotia overwinter on host debris. It is predominantly a problem in warmer soils and not a major issue in Montana. It can cause a lower stem lesion which may weaken the plant. The fungus infects the outer leaf sheath and rots the tissue leaving a characteristic hole. The lesion is circular or elliptical, light brown, and surrounded by a dark brown halo. Sharp eyespot is less able to weaken plants than eyespot and is generally not economical to control.
Variety selection
Plant genetic resistance is not the normal control practice for root and crown diseases. The sole exception is the Pch1 resistance gene for control of eyespot/strawbreaker foot rot. Tolerance of a variety to severe disease is the general practice. In this case, it is best to observe the performance of varieties in side-by-side comparison trials conducted over multiple years and across varied environments. Those varieties that have uniform plant stands and have agronomic yields and test weights in the group of varieties at the top of the test trials will often have the best tolerance to root and crown diseases. Local experiment station trials and private company trials are an excellent source of information on variety performance.
Links to variety performance information for Montana can be found at the Montana State University Plant Sciences and Plant Pathology website, http://plantsciences.montana.edu/mtproducerinfo.html under ‘Producers and Farmers’. The variety selection tool can be found at the Southern Agricultural Research Center’s website at http:// www.sarc.montana.edu/php/varieties.php.
Fungicide Use
Seed treatments have efficacy for approximately two to four weeks after planting. They will have no or only moderate effect against many of the crown rots. Refer to the Montguide, Small Grain Seed Treatment (MT199608AG) for specific recommendations. There is no efficacy data for the use of foliar fungicides to control crown rots in Montana, and in general they are probably not economical. The best management practices for control of root and crown rots include planting date, using the appropriate planting depth, crop rotation, appropriate fertility, and variety selection.
Fungicide recommendations are available from several sources. University sources include fact sheets on the High Plains IPM guide (http://wiki.bugwood.org/HPIPM:Main_Page), AgAlerts, presentations and your county Extension agent. Your fungicide salesperson also has information. Another good source for our region is the NDSU Fungicide Guide at http://www.ag.ndsu.edu/extplantpath/publications-newsletters/fungicides. The majority of the fungicides registered in North Dakota are also registered for use in Montana, but always check the label. Section 18 and 24c (local) registrations are available on the Montana Department of Agriculture website at http://agr.state.mt.us/ pestfert/PesticideRegistration1824.asp.
Other sources of information
Mathre, D. E., ed. 1997. Compendium of barley diseases, 2nd ed. APS Press, St. Paul.
Bockus, W., R. Bowden, R. Hunger, W. Morrill, T. Murray, and R. Smiley. 2010. Compendium of wheat diseases, 3rd ed. APS Press, St. Paul.
Smiley, R.W., A.G. Ogg, and R.J. Cook. 1992. Influence of glyphosate on rhizoctonia root rot, growth, and yield of barley. Plant Dis. 76: 937-942.
Stack, R. and M. McMullen. 1999. Root and crown rots of small grains. North Dakota State University Extension Service Publication PP-785. http://www.ag.ndsu.edu/pubs/plantsci/smgrains/pp785w.htm. Accessed September 2010.
Steffenson, B., J. Pederson, and V. Pederson. 1999. Common barley diseases in North Dakota. NDSU Extension Service publication PP-894. http://www.ag.ndsu.edu/pubs/plantsci/smgrains/pp894.pdf. Accessed September 2010.
Acknowledgments
Partial research support for developing this publication has been provided by the Montana Wheat and Barley Committee.

