
Fungal, Bacterial, and Physiological Leaf Diseases of Cereal Crops (wheat, durum, barley)
Proper diagnosis of leaf diseases will help producers make informed management decisions. In this publication we discuss the symptoms and control of the various leaf diseases common to cereal crops in Montana.
Last Updated: 07/13by Mary Burrows, Extension Plant Pathologist; Jeannie Olmstead, Toole County MSU Extension Agent; and William Grey, Director, Montana Seed Foundation Program
PROPER DIAGNOSIS AND EVALUATION OF THE
potential yield loss due to foliar leaf diseases are some of the most difficult disease-oriented decisions a producer will make. Leaf diseases can be confusing to diagnose because the symptoms of different diseases look very similar.
Tan spot and Septoria leaf spot of wheat
Tan spot and Septoria leaf spot are the two most common wheat leaf diseases in Montana. The adoption of minimum or no tillage is causing an increase in the incidence of these diseases due to increased crop residue on the soil surface. Tan spot is caused by the fungus Pyrenophora tritici-repentis, and Septoria leaf spot by the fungi Septoria tritici or Stagnospora (formerly Septoria) nodorum. Both of these diseases are residue-borne and are controlled in the same manner.
Symptoms of tan spot and Septoria leaf spot occur when weather conditions are moist. In order to distinguish between them, look at the lesion shape and color. Both form very small, yellow spots on the leaves when the fungus first infects the leaf and expand into distinctive patterns. Tan spot lesions are ‘eye’ shaped, sometimes with a brown (necrotic) ‘pupil,’ and have a yellow halo (Figure 1). Tan spot produces a toxin that diffuses from the lesion producing yellow chlorosis that extends beyond from the initial eyespot symptom. Septoria leaf spot lesions tend to be more tan to brown and diffuse to lens-shaped with little to no yellow halo (Figure 1). Lesions may coalesce into a blotch that affects the entire leaf blade. Symptoms will vary with variety and environmental conditions. If you put tan spot or septoria- infected leaves in a moist chamber (a ziploc bag or sealed plastic container with a moist paper towel works well) for two to three days, small black spots will develop in the lesions. These are fungal structures called ‘pycnidia’ and are how the fungus makes more spores and spreads. If no pycnidia form the lesions may be due to physiological leaf spot, which is not caused by an infectious pathogen. Along with the leaf lesions, a glume blotch of the spike can develop if conditions are very moist following heading. The glume blotch affects seed weight and kernel color, causing a red smudge in the case of tan spot.
Both fungi overwinter as spores on cereal stubble and grasses. Spores are ejected from overwintering structures (pycnidia) in the spring and travel through the air to land on nearby susceptible green plant tissue (Figure 2). The fungus requires 6 to 24 hours of moisture in order to infect a leaf, so rain, a significant dew or high canopy humidity are required for fungal infection. Optimal temperatures for symptom development range from 60°F to 80°F. In a dryland situation, after the spring rains stop, spores and symptoms do not spread to the upper leaves of the plant, including the flag leaf.


FIGURE 1. Symptoms of tan leaf spot (left) and Septoria leaf spot (right).
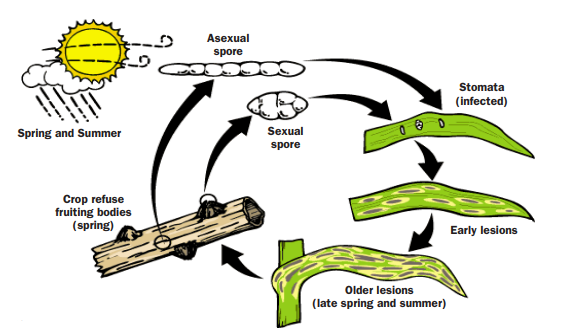
FIGURE 2. Disease cycle for tan spot. (North Dakota State University, 2009)
Tan spot and Septoria leaf spot control
The best methods of controlling tan spot and Septoria leaf spot are cultural: implementing crop rotation to avoid planting into stubble of the same species, tillage (if compatible with your cropping system) to reduce stubble, and using the best yielding variety appropriate for the region. Most varieties of barley are tolerant or resistant to tan spot and Septoria leaf spot. Wheat varieties are available that are resistant to leaf spot. These varieties tend to restrict pathogens to the lower third of the canopy. Foliar fungicides can be useful if significant leaf disease occurs early in the season and the flag leaf is to be protected. General recommendations from North Dakota State University (NDSU) indicate it is not economical to apply a fungicide at tillering or earlier unless you have significant leaf disease. According to research conducted at NDSU the yield reduction due to these diseases will be anywhere from 10-40 percent when the flag leaf is affected. The average yield benefit was 3 bu/acre in NDSU trials when the flag leaf was free of leaf spot diseases. Yield loss will depend on disease severity, variety, and environmental conditions.
The use of foliar fungicides for control of tan spot and Septoria leaf spot on leaves at the tillering stage (vs the flag leaf stage) must be carefully considered due to the need for proper timing of the application, application costs, fungicide effectiveness, and the risk of developing fungicide resistance. Recommendations vary with commodity prices, but a general rule is that yield should be at least 45 bu/a dryland or 75 bu/a irrigated to consider a fungicide in management of the leafspots.
A foliar fungicide trial was conducted by producers in Montana in 2007 and 2008. A half rate of strobulin and triazole fungicide (Stratego [propiconazole + trifloxystrobin, 5 oz/A]) was applied at the tillering stage as a tank mix with a standard herbicide application. Producers in central Montana applied the fungicide to winter wheat, and producers in eastern Montana applied the fungicide to spring wheat (Tables 1 and 2). In both years, cool wet springs favored leaf disease development but significant disease was not seen on the flag leaf. A consistent yield benefit was not observed in either year on winter wheat or spring wheat.
Physiological leaf spot of wheat and barley
Physiological leaf spot, often caused by chloride deficiency, can produce similar symptoms to tan spot and Septoria leaf spot. The pattern of symptoms is on all leaves of the plant, whereas tan spot and Septoria normally start in the lower canopy. With physiological leaf spot, no fungal structures are seen on leaves, even after incubation in a moist chamber, and the lesion edges are sharp (vs. diffuse with fungi). Some varieties are more susceptible to physiological leaf spot than others.
TABLE 1. Yield difference due to fungicide application at tillering on winter wheat in central Montana, 2007 and 2008.
County |
Yield (bu/A) | Irrigation |
|||
| Untreated | Treated | Difference* | |||
| 2007 | Choteau | 84.0 | 88.0 | 4.0 | Dry |
| Choteau | 39.0 | 40.0 | 1.0 | Dry | |
| Choteau | NA | NA | 1.0 | Dry | |
| Choteau | NA | NA | 1.0 | Dry | |
| Choteau | NA | NA | 0.0 | Dry | |
| Liberty | 30.0 | 30.0 | 0.0 | Dry | |
| Liberty | 115.0 | 115.0 | 0.0 | Irrigated | |
| Pondera | 44.5 | 45.5 | 1.0 | Dry | |
| Pondera | 63.0 | 63.0 | 0.0 | Dry | |
| Pondera | 37.0 | 37.0 | 0.0 | Dry | |
| Pondera | NA | NA | 1.5 | Dry | |
| Toole | 31.3 | 31.6 | 0.3 | Dry | |
| Toole | 14.0 | 12.0 | -2.0 | Dry | |
| Toole | 41.0 | 39.0 | -2.0 | Dry | |
| 2008 | Choteau | 63.6 | 64.1 | 0.5 | Dry |
| Liberty | 100.0 | 103.0 | 3.0 | Irrigated | |
| Liberty | 58.0 | 59.0 | 1.0 | Dry | |
| Pondera | 76.0 | 76.0 | 0.0 | Dry | |
| Pondera | 39.8 | 44.2 | 4.4 | Dry | |
| Toole | 38.1 | 38.3 | 0.2 | Dry | |
| Toole | 48.5 | 49.0 | 0.5 | Dry | |
NA = data not available
* Yield difference between treated and untreated (control) plots.
TABLE 2. Yield difference due to early-season fungicide application at tillering on spring wheat in eastern Montana, 2007 and 2008.
County |
Yield (bu/A) | Irrigation |
|||
| Untreated | Treated | Difference* | |||
| 2007 | Daniels | 9.0 | 15.5 | 6.5 | Dry |
| Daniels | 31.4 | 23.8 | -7.6 | Dry | |
| Daniels | 28.2 | 19.2 | -9.0 | Dry | |
| Richland | 27.0 | 29.5 | 2.5 | Dry | |
| Richland | NA | NA | 1.5 | Dry | |
| Roosevelt | 85.0 | 85.0 | 0.0 | Irrigated | |
| Roosevelt | 22.6 | 20.6 | -2.0 | Dry | |
| Roosevelt | 40.0 | 40.0 | 0.0 | Dry | |
| Sheridan | 34.0 | 34.0 | 0.0 | Dry | |
| Sheridan | 30.0 | 30.0 | 0.0 | Dry | |
| 2008 | Daniels | 15.9 | 15.9 | 0.0 | Dry |
| Daniels | 33.0 | 35.0 | 2.0 | Dry | |
| Roosevelt | NA | NA | 0.0 | Dry | |
| Sheridan | 8.0 | 8.0 | 0.0 | Dry | |
| Sheridan | 13.0 | 13.0 | 0.0 | Dry | |
| Sheridan | 31.0 | 32.5 | 1.5 | Dry | |
NA = data not available
* Yield difference between treated and untreated (control) plots.
TABLE 3. Susceptibility to physiological leaf spot in Montana wheat varieties.
| Varieties | |||
| Susceptible | Year released | Resistant | Year released |
| Accipiter* | 2008 | Bond | 2004 |
| Buteo* | 2001 | Bynum | 2005 |
| Falcon* | 1999 | Carter | 2006 |
| Promontory | 1990 | Genou | 2004 |
| Redwin* | 1979 | Hyalite (HWW) | 2005 |
| Wendy (HWW) | 2004 | Jagalene | 2002 |
| Jerry | 2001 | ||
| Ledger | 2004 | ||
| Neeley | 1980 | ||
| Norris | 2005 | ||
| NuDakota (HWW) | 2006 | ||
| NuSky (HWW) | 2001 | ||
| Peregrine | 2008 | ||
| Pryor | 2002 | ||
| Rampart | 1996 | ||
| Rocky | 1978 | ||
| Tiber | 1988 | ||
| Wahoo | 2001 | ||
| Yellowstone | 2005 | ||
*Very susceptible to physiological leaf spot.
FIGURE 3. Physiological leaf spot symptoms can vary signigficantly depending on the variety and environmental conditions.


In general, recently released varieties in Montana are tolerant of physiological leaf spot (Table 3). Symptoms can vary significantly depending on the variety and environmental conditions (Figure 3). Supplementing soil with potash is of limited utility in Montana due to the high soil pH in most areas. Variety selection is the most economical and effective method of controlling these symptoms.
Bacterial leaf blight and black chaff of wheat and barley
Bacterial blight of barley and bacterial stripe of wheat are both commonly referred to as black chaff when on the glumes. These bacterial disease symptoms are often confused with fungal leaf diseases. In Montana, Xanthomonas translucens pv translucens on barley and X. campestris pv translucens on wheat are the most important pathogens. Pseudomonas syringae pv. syringae does cause bacterial leaf blight on both wheat and barley but it is very minor. The leaf symptoms begin as small, water-soaked spots on leaves which elongate into linear streaks that become necrotic tan or brown (Figure 4). Often the tips of the leaves become shredded. Leaf tip shredding can also be caused by drought stress, but in the case of drought stress, the leaf lesions characteristic of bacterial leaf diseases will not be seen on the leaves. The symptoms can also be confused with spot or net blotch or Septoria leaf spot, diseases which are caused by fungi. The leaves of bacterial-infected plants often have a shellac or ‘slick’ feel, whereas leaves infected by fungi do not. Under very wet conditions, beads of bacteria can be exuded from leaves or glumes infected by the bacterial pathogen (Figure 4). Symptoms often appear late in the season or after a hail storm or heavy, driving rain which wounds the plant.
In order to distinguish between bacterial and fungal leaf diseases, one can put leaves in a moist chamber and check for fungal structures (little black dots in the lesions) after two to three days. Also, bacterial lesions will be ‘water-soaked’ or ‘glassy’ before they dry up, particularly if the environment is moist. When in doubt, submit a sample to the Plant Disease Diagnostic Clinic through your county extension agent.
When bacteria infect the head of wheat or barley, it is known as black chaff (Figure 5). Symptoms include pink, tan, or black streaks on the glumes, and discolored and shriveled seed. Under wet conditions an exudate can develop on the glume and seed surfaces forming tiny yellow droplets or a glassy or shellacked appearance.
FIGURE 4. Foliar symptoms of bacterial blight on barley (left) and ooze (right) that occur under wet conditions.


Bacterial diseases can be controlled by planting disease free seed and seed that was grown under dryland conditions. Survival of the bacteria on infested straw is greatly reduced when incorporated into the soil. Symptoms do not generally appear unless the crop is irrigated or moist environmental conditions prevail, but seed can still be infected. Infected seed lots can be tested using laboratory methods. A generally accepted threshold for XCT bacterial populations is 103 cfu/g seed. There are no seed treatments for bacterial diseases. Crop rotation and planting seed free of the pathogen are effective tools to control these diseases.
Net blotch, spot blotch, and scald diseases of barley
Leaf diseases common in barley include net blotch, spot blotch and scald. These are all residue-borne pathogens favored by continuous barley cropping, minimum or no tillage, and irrigation. They can be distinguished based on their symptoms, but controlled using similar techniques. Yield and quality reductions are proportional to the amount of leaf area affected, particularly infection on the flag leaf and the penultimate leaf, or flag leaf minus one. Symptoms will vary according to barley variety, pathogen isolate, and environmental conditions, but generalizations can be made. Symptoms begin as small spots on leaves or stems and expand. Net blotch (Pyrenophora teres) has two forms, one that forms a netlike necrotic symptom and another form that causes a spot symptom on the leaves. Spot blotch (Bipolaris sorokiniana) causes round to oblong, brown lesions surrounded by a chlorotic margin. Scald (Rhynchosporium secalis) can be recognized by its grey or watersoaked lesions with brown margins. Scald lesions have a very distinct dark brown ring around a tan center (Figure 6). All of these diseases can cause a glume blotch and shriveling of the seed. Scald often occurs during the early, cool season, whereas netblotch is more prevalent during the warmer season. Management of these diseases can be achieved by crop rotation, variety selection, irrigation management to reduce of humidity in the canopy, light tillage to reduce residue, and fungicide application.
FIGURE 5. Black chaff on glumes (left) and seed (right).


FIGURE 6. Left to right - net blotch, spot blotch and scald on barley leaves.
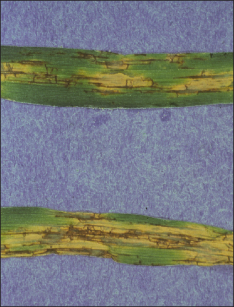

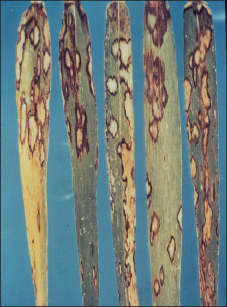
Figure 7. Barley Stripe.

Barley stripe of barley
Barley stripe is a rare disease and easily eradicated as it is a seedborne disease caused by the fungus Pyrenophora graminea. The fungus infects the developing seedling as it germinates and grows inside the vascular system of the plant. Symptoms begin as yellow stripes, particularly on the leaf sheath and the basal portion of the leaf blade (Figure 7). These stripes eventually extend the length of the leaf and become necrotic. They can coalesce and kill the entire leaf. The leaves split and fray at the ends, appearing shredded. Yield loss is proportional to the number of plants which are infected. Control can be achieved by using seed treatments containing imazalil and using clean seed. Do not save seed from affected fields. Foliar fungicides are not effective, since only one cycle of infection and spore production occurs in each season.
Rusts of wheat and barley
Rust diseases are caused by Puccinia spp. and include stripe rust, leaf rust, and stem rust. Spores generally blow in from other wheat-growing areas each year, although stripe rust can overwinter in Montana on volunteer wheat and grassy weeds. Stripe rust occurs early in the growing season, often during seedling or tillering stages. Stripe rust can also develop from over-summering infections when the weather becomes cool and moist in the fall. Leaf and stem rusts will infect late in crop development when spores blow in from other cereal-growing areas of the United States. Rust diseases are favored by moisture. Fungicide application for fall infection is not economical.
FIGURE 8. ‘Stripe rust (upper left), stem rust (upper right) and leaf rust (bottom) symptoms on wheat.



The rusts can be distinguished based on the location of the symptoms, the color of the spores, and the shape of the lesions (Figure 8). All rust diseases are easily recognized by the yellow to reddish or brown pustules that develop on leaves and/or stems. The fungal spores rub off on your finger. Yield loss depends on variety resistance and time of infection. Control is achieved through the use of resistant varieti

