
Preventing and Managing Herbicide-Resistant Weeds in Montana
This Montguide discusses the factors that cause herbicide resistance and describes how to reduce the risk of selecting for herbicide-resistant weed biotypses.
Last Updated: 03/11by Fabian D. Menalled, MSU Extension Cropland Weeds Specialist, and William E. Dyer, MSU Department of Plant Sciences and Plant Pathology
HERBICIDE RESISTANCE IS THE INNATE ABLITY OF
a weed biotype to survive and reproduce after treatment with a herbicide dose that would normally be lethal. In recent years, herbicide-resistant weeds have developed from academic curiosities into serious management issues. While the first account of herbicide resistance occurred in 1957 against 2,4-D, the first confirmed case in Montana was reported in 1984 when kochia plants showed resistance to atrazine. By July 2007, there were 315 cases of herbicide-resistant biotypes comprising 183 species (110 dicots and 73 monocots) throughout the world. Italicized terms in the text are defined in the glossary.
Herbicide resistance in Montana
In Montana, herbicide resistance has been confirmed in four species – kochia (Kochia scoparia), wild oat (Avena fatua), Persian darnel (Lolium persicum), and Russian thistle (Salsola kali) – and is suspected for green foxtail (Setaria viridis).
Is herbicide resistance a real problem?
Herbicide resistance is a serious problem that poses at least three significant challenges for weed management. First, it is very expensive and time consuming to test for herbicide resistance and develop alternative management programs, once resistance has been confirmed. Second, existing herbicides must be protected against resistance development, since very few new products are being released due to their high costs of development. Finally, when producers lose the use of a herbicide because of resistance, as happened for Glean and Telar (chlorsulfuron) to manage kochia in Montana’s small grain production, it limits their weed management options as well as creates serious economic consequences for agriculture.
Luckily, the development and spread of herbicide resistance can be prevented. This article discusses the factors that cause herbicide resistance and describes how to reduce the risk of selecting for herbicide-resistant weed biotypes.
Development and spread of herbicide- resistant kochia in Montana
Kochia resistance to ALS-inhibiting herbicides including Glean and Telar (chlorsulfuron) as well as Ally and Escort (metsulfuron-methyl) was first found in Montana in 1988. Currently, ALS resistant kochia is present in nearly all small grain production areas of Montana, and more than 50 percent of the kochia plants in parts of the Golden Triangle, Yellowstone River Valley and northeastern Montana are resistant.
After the widespread appearance of ALS inhibitor- resistant kochia, most small grain producers used two- and three-way tank mixes of herbicides that included 2,4-D, Banvel (dicamba), and a sulfonylurea or imidazolinone herbicide. However, 2,4-D has never been particularly effective on kochia, leaving only dicamba, a synthetic auxin herbicide, as the main option to control this troublesome species. Unfortunately, in 1995 dicamba-resistant kochia was verified in several areas of Montana. More recently, Starane (fluroxypyr) -resistant kochia was found in northern Montana. These dicamba-and fluroxypyr-resistant kochia populations don’t seem to be spreading as fast as ALS inhibitor-resistant kochia.
Where do resistant weeds come from?
Weeds, like every other organism, have inherent genetic variability that arises from mutations. It is this genetic variability that allows one or a few plants already present within a population, usually at a very low frequency (maybe one in several millions) to survive herbicide treatment. It is very important to note that the frequent use of a herbicide does not cause the genetic change that allows resistance. Instead, it creates the selection pressure that favors the spread of resistant biotypes (Figure 1).
FIGURE 1. Where do resistant weeds come from?
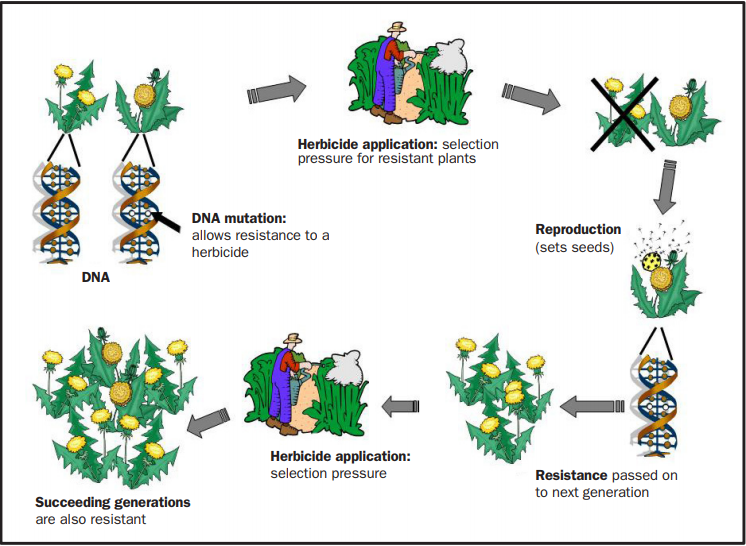
Growers do not usually notice resistant plants during the first few years of their appearance, or attribute them to application problems. However, by repeatedly using the same herbicide over time, you remove all susceptible individuals and select for the resistant plants. Then, depending on the selection intensity and life history of the weed species (see below), the resistant weed population will continue to grow and expand. Unfortunately, most growers do not recognize these infestations until about 25 percent of the weeds in a given field are resistant.
Types of resistance
Weeds that are resistant to one herbicide may also be resistant to other herbicides in the same family. In other words, all members of a herbicide family target the same biochemical site in the plant. For example, a biotype of wild oats that is resistant to Hoelon (diclofop), an ACCase inhibitor, may be resistant to other ACCase inhibitors such as Achieve (tralkoxydim). This is known as cross-resistance. On the other hand, multiple resistance occurs when a biotype is resistant to more than one family of herbicides with different biochemical targets, such as triazines and ALS inhibitors. To control weeds with multiple resistance, use herbicides that are not in any herbicide family to which there is resistance, or use a non- herbicide control strategy.
Herbicide-resistant wild oats in Montana
In 1990, after 15 to 20 years of continuous use of the herbicide Fargo (triallate), resistant wild oat plants began to appear on farms on the Fairfield Bench. Unfortunately, these plants are also cross-resistant to Avenge (difenzoquat), an unrelated wild oat herbicide. Many affected producers switched to Assert (imazamethabenz- methyl), an alternative herbicide with a different mode of action, and this strategy was effective for five years or so. However, Assert-resistant wild oats were identified in the state in 1996. In addition, some wild oat populations show multiple herbicide resistance.
TABLE 1. The effects of agronomic practices, weed characteristics and herbicide properties on the risk of developing herbicide- resistant weeds.
| -------- Risk of resistance -------- | |||
| Low | Intermediate | High | |
Weed control practices |
Integration of cultural, mechanical and chemical | Mechanical and chemical | Chemical |
| Crop rotation | Diversified rotation | Limited rotation | Monoculture |
| Weed abundance | Low | Intermediate | High |
| Weed susceptibility to a particular herbicide | Low | Intermediate | High |
| Weed seed longevity | Long | Intermediate | Short |
| Weed reproduction mechanism | Cross-pollination | Cross- and self-pollination | Self-pollination |
| Number of herbicide modes of action used per season | > 2 | 2 | 1 |
| Number of site of action targeted by the herbicide | Multiple | Few | Single |
| Herbicide soil residual period | Short | Intermediate | Long |
Resistance mechanisms
There are three main mechanisms by which weeds can become resistant to a herbicide.
1. The resistant plant can have an altered target site, which means that the herbicide no longer binds to its normal biochemical target due to a structural change.
2. Enhanced metabolism refers to the ability of a resistant plant to degrade a herbicide to nontoxic byproducts.
3. Compartmentalization or sequestration occurs when the herbicide is removed from sensitive sites in the plant to tolerant locations where it is essentially harmless.
Factors controlling the development of resistant weeds
Three factors determine the chances that a given weed species will become resistant to a particular herbicide (Table 1).
1. Selection pressure. If a herbicide is highly effective, applied often, has long soil residual activity and is the only practice for controlling a particular weed, then the selection pressure for herbicide resistance is very high. Under these conditions, selection for resistant weeds can occur in only a few years.
Perhaps the easiest way to decrease the selection pressure for resistant weeds is to rotate crops. Rotating crops translates into a diverse set of chemical and non-chemical weed management practices, making it difficult for herbicide-resistant weeds to survive and increase in abundance.
2. Weed biology. Some weed species have high levels of genetic variability, meaning that a single species consists of many different varieties or biotypes. Generally, weeds like kochia that are cross-pollinated have more diversity than those that are self-pollinated like wild oats. Weeds with more genetic variability generally develop resistance to herbicides sooner, since the initial frequency of resistant biotypes before spraying is probably higher. In contrast, weeds with long-lived seeds tend to develop resistance more slowly, since susceptible seeds from the seedbank germinate over many years and add susceptible plants to the population.
3. Genetics of resistance. Differences among the various herbicide target sites can dictate whether resistance is more or less likely to develop. Some target sites that are more variable tend to develop resistance sooner, since this variation creates a higher incidence of resistant plants. For example, resistance to ALS inhibitors developed within three or four years of continuous use of these herbicides because this target is highly variable. In contrast, other targets like EPSP synthase, the site of Roundup (glyphosate) toxicity, do not mutate so often. Resistance to glyphosate has only occurred in a few instances and took many years to develop.
Diagnosing herbicide resistance
The failure of a herbicide to control weeds does not necessarily mean that the weeds are herbicide-resistant. Before drawing this conclusion, consider other possible factors: application problems, unfavorable weather, improper timing and late weed emergence. Only after ruling out these possibilities should producers suspect resistance is the problem. The following questions can help determine if weed resistance is actually present.
1. Is the level of control different for all species listed on the label? It is highly unlikely that several species will develop resistance at the same time. If uncontrolled weed patches contain several different species, then it is likely that the lack of control is due to reasons other than resistance.
2. Has the same herbicide been used in the same field for several consecutive years? Multiple applications of herbicides with the same site of action will increase the chances of selecting resistant biotypes.
3. Are there other cases of herbicide-resistant weeds in the local region?
4. Has the level of weed control on a particular species declined in recent years?
If the answer to one or more of these questions is “yes” and all other factors have been ruled out, then resistance may be the problem. Ideally, to test this out, plants started from seeds collected from suspected resistant plants should be grown in a greenhouse and then treated with the herbicide. In any case, switch to another herbicide with a different site of action, or to a non-chemical weed management practice. You should also contact your local MSU Extension agent or weed specialist to test for resistance, and to develop a comprehensive herbicide resistance management program.
Strategies for avoiding and managing herbicide-resistant weeds
Preventing and managing herbicide-resistant weeds require an integrated approach for controlling weeds. In addition to using herbicides, your program should include all possible biological, cultural and mechanical weed control practices. Do not rely too heavily on any one method. The following management practices will help reduce the selection pressure for resistant weeds:
1. Minimize your reliance on herbicides. If possible, use herbicides based on actual (not predicted) weed infestations and use site-specific technology to make applications only where weed numbers exceed economic thresholds.
2. Integrate different management practices. Combine, whenever possible, biological, mechanical and cultural weed control practices with herbicides.
3. Rotate herbicides. Do not make more than two consecutive applications of herbicides with the same site of action in the same field, unless other effective control practices are included. For example, Ally and Harmony GT (sulfonylurea) as well as Pursuit and Assert (imidazolinone) herbicides target the same plant enzyme, called ALS. Continuous use of one of these herbicides could lead to the evolution of weed biotypes that are resistant to most or all herbicides that target ALS. Recently, producers have begun using Osprey (mesosulfuron-methyl), an ALS- inhibitor herbicide, to control Persian darnel (Lolium persicum). This herbicide provides similar control to Discover (clodinafop- propargyl) and Achieve, both ACCase inhibitors herbicides. Thus, Osprey can be used to manage ACCase-resistant Persian darnel. Also, by rotating it with Discover or Achive, it can help reduce the risk of resistance development.
4. Use herbicide mixtures. Apply herbicides in tank- mixed, prepackaged or sequential mixture, and combine products with different modes of action. It is important that each herbicide used in a mixture has significant activity against potentially resistant weeds (see below for tips on selecting tank-mixing partners).
5. Rotate crops with different characteristics to break up weed life cycles. For example, a rotation that includes winter wheat, alfalfa, and summer crops such as spring wheat, barley, corn or dry beans creates different environmental conditions and stresses weeds. Also, crop rotation allows you to rotate different herbicides and management practices.
6. Scout your fields for resistant weeds. Walk your fields before and shortly after herbicide applications and quickly destroy weed escapes using alternative measures.
7. Use herbicides with short soil residues. In general, herbicides with a long soil residue prolong the pressure to select for resistant weeds. Increasing the dose of a residual herbicide will usually extend its residual period in the soil.
8. Clean your equipment to prevent the spread of resistant biotypes. Minimize the chances of disseminating resistant weeds by cleaning tillage and harvest equipment before moving them from infested fields to clean fields.
9. Be careful when using herbicide-resistant crops. Plant- ing herbicide-resistant crops should not translate into the continuous use of the same herbicide. It is still just as im- portant to rotate herbicides and other control measures.
10. Make postharvest weed control part of your regular field practices. Many weeds can exist unnoticed under a crop canopy, but have enough time to set viable seed once the crop is removed. Postharvest treatments can dramatically reduce weed seed production and prevent worse infestations the following year.
Tips for selecting tank-mixing partners
- All herbicides in the tank-mix must have different modes of action.
- They should be equally effective in killing the same spectrum of weed species.
- All herbicides should have similar soil persistence.
TABLE 2. List of herbicides grouped by site of action, herbicide families and example of resistant weeds found in Montana1.
| Site of action | Chemical family | Common name | Trade name(s) | Resistant weeds found in Montana2 |
ACCase inhibitors - prevent formation of fatty acids |
aryloxyphenoxy propanoates |
clodinafop | Discover | Wild oat Persian darnel |
| diclofop | Hoelon | |||
| fenoxaprop | Puma, Acclaim | |||
| fluazifop | Fusilade DX | |||
| quizalofop | Assure II | |||
cyclohex- anediones |
clethodim | Prism, Select 2 EC | ||
| sethoxydim | Poast | |||
| tralkoxydim | Achieve | |||
ALS inhibitors - block protein synthesis |
imidazolinones |
imazamethabenz | Assert | Kochia, Russian thistle, wild oat |
| imazamox | Raptor | |||
| imazapic | Plateau | |||
| imazapyr | Arsenal | |||
| imazaquin | Scepter | |||
| imazethapyr | Pursuit, Lightning | |||
sulfonylamino- carbonyltriazol- inones |
flucarbazone-sodium | Everest |
||
| propoxycarbazone-sodium | Olympus |
|||
sulfonylureas |
chlorsulfuron | Glean, Telar, Finesse | ||
| ethametsulfuron | Muster | |||
| halosulfuron | Permit | |||
| metsulfuron | Ally, Escort | |||
nicosulfuron |
Accent, Accent Gold, Basis3, Basis Gold3, Celebrity Plus | |||
| primsulfuron | Beacon, Exceed3 | |||
| prosulfuron | Peak, Exceed | |||
rimsulfuron |
Matrix, Accent Gold3, Basis Gold3 | |||
| sulfometuron | Oust | |||
| sulfosulfuron | Maverick |
Site of action |
Chemical family |
Common name |
Trade name |
Resistant weeds found in Montana2 |
ALS inhibitors - block protein synthesis |
sulfonylureas |
thifensulfuron |
Harmony GT, Basis3, Harmony Extra3 | Kochia, Russian thistle, wild oat |
| triasulfuron | Amber, Rave | |||
| tribenuron | Express, Harmony Extra3 | |||
| triflusulfuron | UpBeet | |||
triazolopyrimides |
chloransulam | FirstRate | ||
| diclosulam | StrongArm | |||
| flumetsulam | Broadstrike | |||
Mitosis inhibitors - Interfere with new plant growth |
dinitroanalines |
benefin | Balan, Team | |
| ethalfluralin | Sonalan | |||
| oryzalin | Surflan | |||
| pendimethalin | Prowl, Pendimax, Squadron3, others | |||
| trifluralin | Treflan, others | |||
Synthetic auxins - growth regulators |
phenoxy acetic acids |
2,4-D |
2,4-D, Campaign3, Crossbow3, Curtail3, Landmaster BW3, Shotgun3, Starane Salvo3, Weedmaster, others | Kochia(not necessarily to all these hebicides) |
| 2,4-DB | Butyrac | |||
| MCPA | MCPA, others | |||
| benzoic acid | dicamba | Banvel, Clarity | ||
pyridines |
clopyralid fluroxypyr picloram | Stinger, Reclaim StaraneTordon 22K | ||
| quinolines | quinclorac | Paramount, Drive | ||
Photosystem II inhibitors -block photosynthesis |
triazines |
atrazine | Aatrex, others | Kochia |
| cyanazine | Bladex | |||
| simazine | Princep | |||
triazones |
hexazinone | Velpar | ||
| metribuzin | Sencor | |||
uracils |
bromacil | Hyvar | ||
| terbacil | Sinbar |
Site of action |
Chemical family |
Common name |
Trade name(s) |
Resistant weeds in Montana2 |
| Photosystem II inhibitors – block photosynthesis(different binding behavior than groups 5 and 7 but same site of action) | benzothiadiazoles | bentazon | Basagran, Storm3, Laddok3 | |
nitriles |
bromoxynil |
Buctril, Moxy, Broclean, Bronate, others | ||
| phenyl-pyradazine | pyridate | Tough | ||
| Photosystem II inhibitors – block photosynthesis (different binding behavior than groups 5 and 6 but same site of action) | amide | propanil | Stampede | |
ureas |
diuron | Diuron, Direx, Karmex | ||
linuron |
Lorox, Linex |
|||
Lipid synthesis Inhibitors, but not ACCase inhibitors |
thiocarbonates |
cycloate | Ro-Neet | Wild oat |
| EPTC | Eptam | |||
| EPTC + safener | Eradicane | |||
| triallate | Far-Go | |||
| Unknown site of action | no family name | difenzoquat | Avenge | Wild oat |
EPSP synthase inhibitors – block protein synthesis |
glyphosate |
glyphosate |
Roundup, Rodeo, Glyphomax, Backdraft3, Campaign, Extreme, Landmaster BW3, FallowMaster3, ReadyMaster ATZ3, Touchdown | |
| Glutamine synthetase inhibitors – ammonia assimilation inhibitor | phosphorylated amino acid | glufosinate |
Liberty, Finale, Rely |
|
PPO inhibitors – cell membrane disruptor |
diphenylether | fomesafen | Flexstar, Reflex | |
| N-phenylthalimides | flumiclorac | Resource, Stellar | ||
triazolinones |
carfentrazone | Aim | ||
| sulfentrazone | Authority, Spartan, Canopy XL3 | |||
Unknown site of action |
chloroacetamides |
acetochlor | Harness, Surpass, TopNotch | |
| alachlor | Lasso | |||
| dimethenamid | Frontier | |||
| metalochor | Dual II, Magnum | |||
| propachlor | Ramrod | |||
| Photosystem I electron diverters – cell membrane disruptor | bipyridiliums |
diquat | Reglone | |
| paraquat | Gramoxone Extra, Starfire |
Glossary
Biotype: A group of plants within a species that has certain biological traits different from the rest of the population. In most cases, biotypes are not easily recognizable by casual observation.
Cross-pollination: The transfer of pollen from one plant to another by insects or wind.
Herbicide mode of action: The entire sequence of events that occurs from absorption of the herbicide until the plant dies.
Herbicide site of action: The biochemical site within the plant where the herbicide exerts its toxic effects.
Metabolism: Metabolic reactions may break down herbicides into nontoxic by-products. Most crop insensitivity to selective herbicides is due to enhanced rates of metabolism.
Mutation: A specific change in the genetic makeup of the plant that can lead to a change in its appearance or sensitivity to a herbicide.
Selection pressure: A measure of the effectiveness of a herbicide in killing weeds. It includes factors like efficacy, soil residual period and how often the herbicide is applied.

